Abstract
The effects of smoking marijuana on regional cerebral blood flow (rCBF) and cognitive performance were assessed in 12 recreational users in a double-blinded, placebo-controlled study. PET with [15Oxygen]-labeled water ([15O]H2O) was used to measure rCBF before and after smoking of marijuana and placebo cigarettes, as subjects repeatedly performed an auditory attention task. Smoking marijuana resulted in intoxication, as assessed by a behavioral rating scale, but did not significantly alter mean behavioral performance on the attention task. Heart rate and blood pressure increased dramatically following smoking of marijuana but not placebo cigarettes. However, mean global CBF did not change significantly. Increased rCBF was observed in orbital and mesial frontal lobes, insula, temporal poles, anterior cingulate, as well as in the cerebellum. The increases in rCBF in anterior brain regions were predominantly in “paralimbic” regions and may be related to marijuana's mood-related effects. Reduced rCBF was observed in temporal lobe auditory regions, in visual cortex, and in brain regions that may be part of an attentional network (parietal lobe, frontal lobe and thalamus). These rCBF decreases may be the neural basis of perceptual and cognitive alterations that occur with acute marijuana intoxication. There was no significant rCBF change in the nucleus accumbens or other reward-related brain regions, nor in basal ganglia or hippocampus, which have a high density of cannabinoid receptors.
Similar content being viewed by others
Main
Marijuana (Cannabis sativa) has been the most widely used illicit drug in the United States for a number of decades. Following steady declines throughout the late 1970s and the 1980s, the prevalence of marijuana use among youth skyrocketed in the early and middle 1990s, and has remained high (SAMHSA 1998, 1999; Johnston et al. 1999). Marijuana is typically smoked, resulting in subjective effects that may include euphoria, depersonalization, altered time sense, lethargy, drowsiness, confusion, and anxiety (Solowij 1998). Smoking marijuana may also result in impairment on sensory, motor, and cognitive tasks (Block et al. 1992; Heishman et al. 1990).
Over 60 cannabinoids have been identified in the plant Cannabis sativa, but delta-9-tetrahydrocannabinol (THC) appears to be the major psychoactive ingredient of marijuana (Harvey 1999). THC has a long biological half-life (4–12 days) because of extensive accumulation of the drug in fatty tissue (Huestis 1999). Two types of cannabinoid receptors have been identified in mammals, CB1 and CB2 (Pertwee 1997). There have been suggestions that one function of the cannabinoid system is to modulate dopaminergic (DA) activity (Biegon and Kerman 1995; Loeber and Yurgelun-Todd 1999). Cannabinoid receptors have a distribution in the brain that is similar to that of DA (high concentrations in the basal ganglia, hippocampus), but there is also a high density of cannabinoid receptors in the cerebellum (Herkenham 1992). High density bands of cannabinoid receptors have also been found in the prefrontal cortex in human post-mortem tissue, particularly around the cingulate and superior frontal gyri (Biegon and Kerman 1995).
The few imaging studies that have assessed the effects of marijuana and THC on brain blood flow and metabolism in humans have produced conflicting results. An early single photon emission computerized tomography (SPECT) study by Mathew and colleagues using the 133Xenon inhalation technique compared experienced (a minimum of 10 marijuana cigarettes a week for 3 years), and inexperienced marijuana users before and after smoking marijuana or placebo (Mathew et al. 1989). In both groups the effects of smoking placebo were similar to the effects of smoking marijuana. However, the inexperienced smokers showed a decrease in global cerebral blood flow (gCBF) that was significantly greater for marijuana than placebo, whereas the experienced smokers showed increased CBF that was the same following marijuana and placebo. In a later 133Xenon SPECT study by Mathew and colleagues (Mathew et al. 1992) assessed regional cerebral blood flow (rCBF) following smoking marijuana cigarettes of two different potencies and placebo in 20 experienced male smokers. Smoking marijuana increased global CBF significantly more than placebo, and the greatest increases in rCBF were in the frontal lobes and in the right hemisphere. Mathew et al. (1997) have more recently reported rCBF measurements obtained in 32 normal volunteers using PET with [15O]H2O before and after intravenous (I.V.) infusion of either of two doses of THC or a placebo, given under double blind conditions. THC resulted in increased rCBF bilaterally in the frontal lobes, the insula, anterior cingulate and in subcortical regions, with the largest effects in the right hemisphere.
Volkow et al. (1991a,b) assessed regional glucose metabolism (rGluM) with PET and [18F]FDG following THC injection in eight male volunteers who were occasional users of marijuana (a mean of one marijuana cigarette every two months). Volkow et al. found a great deal of individual variability in the changes in cerebral glucose metabolism induced by THC. Three of eight subjects in their study showed an increase, three showed a decrease, and two had no change in global metabolism in the cerebrum. However, all subjects showed an increase in normalized metabolism in the cerebellum (12% increase over baseline), and the cerebellar increase had a significant correlation with subjective ratings of intoxication. These findings are consistent with the high density of cannabinoid receptors in the cerebellum (Herkenham et al. 1990). Volkow et al. (1996) described the cerebral metabolic changes induced by THC in eight chronic users of marijuana and eight non-users. At baseline, the chronic marijuana users had significantly less relative metabolic activity in the cerebellum than did normal control subjects. Cerebellar activity was increased in both non-users and chronic users of marijuana, but only the chronic group showed significant increases in orbitofrontal cortex, prefrontal cortex and basal ganglia.
The brain imaging studies reviewed above all utilized a “resting” baseline condition in which subjects perform no explicit task when the effects of marijuana or THC on the brain are assessed. Studies of the resting state represent an important first step in understanding the behavioral and physiological effects of marijuana. However, assessing the effects of marijuana only in a resting state confounds the direct effects of marijuana on cerebral function with changes in subjective experience and cognitive state that result from intoxication. Recent PET studies carried out in our laboratory measured rCBF using [15O]H2O prior to and following smoking of a marijuana cigarette, and controlled the subjects’ mental activities by engaging them in a auditory attention task (O'Leary et al. 2000). This initial study of five subjects found that smoking marijuana increased rCBF in a number of “paralimbic” brain regions (e.g., orbital frontal lobes, insula, temporal poles) and in anterior cingulate and cerebellum. Large reductions in rCBF were observed in temporal lobe regions that are sensitive to auditory attention effects.
The present study involved a second, independent sample of 12 volunteers who were occasional recreational smokers of marijuana. Unlike the study reported in O'Leary et al. (2000), the present study included the use of a placebo cigarette (marijuana with the THC removed), as well as an active marijuana cigarette, and utilized a double blind design. We recruited 12 new subjects who were assessed with PET before and after smoking either marijuana or placebo cigarettes in a single 8-injection study. Arterial lines were placed in all subjects to permit calculation of quantitative cerebral blood flow.
METHODS
The subjects were 12 healthy volunteers (6 males, 6 females, mean age 30.5, sd = 8.6 years) who were occasional recreational users of marijuana. They reported their current use of marijuana as no more frequently than 10 times a month (mean = 2.7 times a month over the past year), and had an average duration of usage of six years. All of the subjects were right handed. Subjects were asked to refrain from smoking marijuana for seven days prior to the PET study. A urine screening test (TRIAGER Drugs of Abuse Panel Kit) was administered to all subjects on the day of their PET scan. All subjects had a negative value for THC and six other drugs of abuse, indicating that they had not smoked marijuana for at least four days prior to the study. All subjects provided written consent in compliance with the guidelines of the University of Iowa Institutional Review Board and Radiation Protection Committee.
As detailed in Table 1, there were nine PET conditions. An initial “scout” condition was used to familiarize subjects with procedures and to measure arrival time of the bolus of [15O]H2O in the brain. This was followed by eight PET image acquisition conditions. The scout and first PET imaging condition utilized an auditory choice reaction time (RT) task, which matched the dichotic tests in number of trials, durations of auditory stimulation, and motor responses. It utilized easily discriminable, binaurally presented stimuli, consisting of 21 pure tones (1319 Hz, 500 ms duration), randomly mixed with 104 bursts of white noise (500 ms duration). Subjects responded to each tone by pressing a button with their right thumb as soon as they detected a target tone.
For the dichotic conditions, subjects were instructed to attend only to their left ear. On each trial, pairs of nonsense words were delivered simultaneously to the left and right ears via foam insert earphones at 80 dB SPL. The nonsense words were digitized natural speech, about 500 ms long. The initial and final consonants were stops (i.e., /p/, /b/, /d/, /t/, /g/, and /k/), which were combined with one of five vowels. Each trial consisted of 125 pairs of stimuli presented in random order with an interstimulus interval of 800 ms, including 21 trials with the target presented to the attended ear and 10 with the target presented to the unattended ear. Subjects responded to the target in the attended ear by pressing a button with their right thumb as fast as possible. Responses were scored as hits if they occurred within 200–2000 ms after a target presented to the attended ear. Responses and reaction times were recorded by a personal computer. During the PET session, testing for both the dichotic tests and the control test began 70 s before the estimated time of bolus arrival in the brain and lasted for an additional 100 s.
After the first dichotic condition a marijuana or placebo cigarette was smoked (half of the subjects smoked marijuana first and half placebo). Both marijuana and placebo cigarettes were obtained from the National Institute of Drug Abuse. The marijuana cigarettes contained a moderate amount of THC (∼20 mg), and the placebo contained marijuana with the THC removed. Neither the subject nor the staff in the PET imaging suite at that time knew which cigarette contained active THC or was the placebo. The subject smoked the cigarettes, which were held in a hemostat, while in a reclined position on the PET couch. A paced smoking procedure similar to that described in Block et al. (1992) was used with subjects inhaling for 5 s, holding the smoke in their lungs for 5 s, and then exhaling. The subjects rested for 25 s and were then again told to inhale, hold and exhale. This continued until the cigarette was too small to be smoked. Subjects exhaled into a dome- shaped device suspended about 12 inches above their head and the smoke was vented from the PET camera room.
Following smoking, subjects were repositioned in the PET camera by lining up marks drawn on their skin and laser guide lights. Subject's heads were constrained only lightly by tape but images from each PET condition were co-registered with each individual's MRI image before analysis as described below. If subjects could not be adequately realigned another transmission scan was performed for attenuation correction of the PET images from the remaining conditions (see below). After smoking the initial cigarette, the subject performed three dichotic conditions with PET imaging at approximate 15 min intervals. A second cigarette was then smoked, the subject repositioned, and three more dichotic conditions with PET imaging were conducted.
A “highness” rating scale (0 “not at all”, 10 “highest ever”) was administered verbally after each PET image acquisition and immediately after each smoking condition. The Beck Anxiety inventory was also administered at a preliminary session, and after the first, fourth, and seventh PET image acquisitions. Heart rate and blood pressure were recorded after each PET image acquisition and immediately after each smoking session. Two blood samples were obtained from the arterial line after each PET image acquisition and immediately following each smoking session. One of the blood samples was placed on ice and immediately delivered to the University of Iowa Hospitals and Clinics Specimen Control Laboratory for analysis of total carbon dioxide (TCO2), partial pressure of CO2 and carboxyhemoglobin. The other sample was immediately centrifuged. From this sample plasma was pipetted, placed in a freezer at –20 C, and later sent as a batch to NIDA's Radioimmunoassay Laboratory in Research Triangle Park, North Carolina for analysis of THC levels.
PET Data Acquisition
Each subject had an arterial catheter placed in the radial artery of one arm for blood sampling and a venous catheter in the antecubital vein of the other arm for injection of the [15O]H2O. They were then positioned in the PET camera. Laser guide lights were used to align the subject so that the most caudal slice was aligned with the auditory meatus and the outer canthus of the eyes. The subjects’ heads were lightly taped and marks were drawn on their skin for use in realignment prior to each image acquisition. A rotating pin source of [68GE]germanium was used to acquire a transmission scan for attenuation correction of the emission images. The scout condition used a 15 mCi dose and the remaining conditions utilized 50 mCi of [15O]H2O. PET image acquisition conditions were repeated at approximately 15 min intervals except for the conditions following smoking. Smoking the THC and placebo cigarettes took approximately 15–20 min and PET imaging commenced approximately 10–15 min following smoking. Subjects remained in the PET Center following the study until their heart rate and blood pressure returned to baseline, they reported no symptoms of anxiety, and reported that they were no longer intoxicated (a highness rating of 3 or less). They were then placed in a cab and driven to their home and were asked to remain at home for the remainder of the day.
Regional cerebral blood flow (rCBF) was measured using the bolus [15O]H2O method with a GE4096PLUS Scanner (Herscovitch et al. 1983; Hichwa et al. 1995). For each emission scan, fifteen slices (6.5 mm center-to-center) were acquired with a 10 cm axial field of view. Dynamic imaging and arterial blood sampling were acquired over a 100-s interval following venous injection. The dynamic imaging data were summed from the 40 s immediately following bolus transit, determined by time-activity curves from a region of interest over a cerebral artery. The summed images and arterial blood samples were then used to calculate tissue perfusion in mL/min/100 g tissue using the autoradiographic method (for details see Hurtig et al. 1994; Wollenweber et al. 1997). Quantitative flow images were processed by the Image Processing Laboratory (IPL) of the Iowa Mental Health Clinical Research Center.
MR Imaging
The MR images were acquired for each subject in a 1.5 Tesla General Electric Signa scanner using an SPGR sequence, flip angle of 40 degrees, TE of 5 ms, TR of 24 ms, and with 2 NEX. The contiguous 1.5 mm thick coronal slices were processed by the IPL using locally-developed BRAINS software (Andreasen et al. 1993).
PET and MRI Processing
Each subject's MR images were processed utilizing a combination of automated methods and hand editing, resulting in a brain that was aligned in a standardized coordinate space. (Talairach and Tournoux 1988; Cizadlo et al. 1994). The outlines of the PET images were automatically identified with an edge detection algorithm and the PET images for each condition for each subject were co-registered with their MR images using a variance minimization program (Woods et al. 1992). An 18 mm Hanning filter was applied to the PET images for each condition to eliminate residual anatomical variability.
Following spatial normalization and filtering, within-subject subtractions were computed to compare a number of different conditions. The subtractions were followed by across-subject averaging of the subtraction images and computation of voxel by voxel t-tests of the rCBF changes. Significant regions of activation were calculated on the t-map images, using a technique that corrects for the large number of voxel by voxel t-tests performed, the lack of independence between voxels, and the resolution of the processed PET images (Worsley et al. 1992).
The pre-smoking auditory RT condition was subtracted from the pre-smoking dichotic condition and from the conditions following smoking of THC and placebo. These subtractions highlight the cognitive activation resulting from the dichotic listening task pre- and post-smoking and permit a qualitative assessment of attention-related changes in activation due to smoking marijuana and placebo. A second set of analyses subtracted the pre-smoking dichotic condition from the dichotic conditions following smoking of marijuana and placebo. These analyses allowed assessment of the effects of smoking marijuana and placebo during the performance of the same cognitive task. Finally, subtraction of the post-smoking placebo condition from the post-smoking marijuana condition allowed assessment of the effects of THC on rCBF controlling for the specific effects of smoking and the cognitive task.
RESULTS
Behavioral and Physiological Data
Prior to smoking, the mean subjective rating of the magnitude of intoxication or “high” (10 = “highest ever”) was 0; the mean ratings (and standard deviations) for the three conditions following smoking of the marijuana cigarette were: 7.4 (1.7), 7.3 (1.5), 7.0 (1.8). The ratings following placebo were: 4.7 (3.6), 4.4 (3.6), 3.5 (3.7). Since half of the subjects smoked the marijuana cigarettes first, the highness ratings following placebo for these subjects were influenced by residual marijuana effects. This is indicated by the fact that the six subjects who smoked placebo first had mean ratings of 1.8, 1.6, 0.7 for the three PET conditions following smoking of the placebo cigarette.
The subjects performed extremely well on the baseline auditory RT task (mean = 666.8 ms, sd = 109.4) and on the dichotic tasks, with over 98% correct detections of left ear targets in every dichotic condition. Mean reaction time for the dichotic tasks was 887.9 ms (sd = 148.1) for the pre-smoking dichotic condition. For the three conditions following smoking marijuana the RTs were: 845.4 (155.6), 914.0 (163.4), and 923.2 (195.4) ms. The RTs for the three conditions following smoking of placebo were: 911.3 (226.4), 975.3 (216.7), and 986.0 (228.8) ms. Paired t-tests indicated that none of the RTs for the post-smoking conditions differed significantly from the pre-smoking RT.
As can be seen in Table 2, mean heart rate (HR) increased dramatically following smoking of marijuana but not placebo cigarettes. Compared with the pre-smoking dichotic condition (66.2 beats/min) HR increased significantly in the PET condition following smoking marijuana (106.8 beats/min) t = −6.1, p < .001, as well as in the next two conditions (93.5 and 82.6 beats/min respectively, with t = −5.3, p < .001, and t = 4.5, p < .001). HR changes in three conditions following smoking placebo (75.4 (12.0), 72.6 (121.3), 70.2 (11.1)) were non-significant in comparison to the pre-smoking dichotic condition.
Blood pressure also increased after smoking marijuana, but the changes were more variable across individuals and less dramatic. In the PET condition following smoking of marijuana diastolic pressure was significantly higher than in the pre-smoking condition (pre-smoking mean = 69.2 mm Hg (sd = 7.1), post-smoking mean = 79.2 mm Hg (sd = 14.5), t = −2.7, p < .02). Diastolic pressure remained significantly higher in the second post-marijuana condition (mean = 76.9 mm Hg (sd = 9.4), t = −3.3, p < .007) than in the pre-smoking condition, but was no longer significantly higher in the third post-marijuana condition (70.5 mm Hg (sd = 8.3) ns). After smoking placebo, diastolic pressure was not significantly different from the pre-smoking condition at any time point. Systolic pressure was also not significantly higher after smoking marijuana or placebo for any condition.
The mean (and standard deviation) values for THC in plasma are given in Table 2. These were obtained from blood samples obtained immediately after the completion of PET imaging for each of the post-smoking conditions. A value of 122.1 ngr/mL (sd = 104.3) was measured in a sample that was obtained immediately following smoking of the marijuana cigarette. This dropped quickly to the tabled value of 37.1 ng/mL observed in the sample obtained after the first PET acquisition following smoking, which occurred approximately 15 min later. Thus, THC levels drop off rapidly in the first few minutes following smoking. We were unable to observe the acute effects of these high THC levels on rCBF because of the time required to reposition the subject in the scanner following smoking.
Table 3 lists the correlations (Pearson) of THC levels with behavioral task performance (reaction time), the highness rating, heartrate, and blood pressure. Behavioral performance did not correlate significantly with THC levels, perhaps because of the large variability in the plasma THC measure (see standard deviations of THC in Table 2). Subjective ratings of “highness” also did not correlate significantly with THC levels, except for the rating that immediately followed smoking the placebo cigarette. Except for the assessment immediately following smoking marijuana, heart rate showed a consistently significant relationship with THC level, but blood pressure did not.
PET Data
Whole brain PET counts (a measure of the concentration of the radiotracer in the brain) dropped following smoking of marijuana in comparison to the pre-smoking condition (from 940 to 707, t = 4.9, p < .0001, see Table 2), and remained significantly lower for the next two conditions (t = 3.6, p < .004 and t = 3.4, p < . 006). PET counts were also lower in the first condition following smoking placebo (mean = 856, t = 2.6, p < .03), but this appeared to be a carryover effect from smoking marijuana. The six subjects who smoked marijuana first had a mean PET count of 810 for the condition following smoking placebo, whereas the six subjects who smoked placebo first had a mean PET count of 902 following placebo. All 12 subjects showed a pattern of increased HR and decreased PET counts when comparing the conditions prior to and following smoking marijuana. In contrast, mean whole brain blood flow (WBBF) did not change significantly following smoking of either marijuana or placebo cigarettes. In the condition immediately prior to smoking marijuana, mean WBBF was 51.9, and was 49.6 ml/min/100 g following smoking. Six of the subjects had increased WBBF and half had decreased WBBF following smoking marijuana.
A t-threshold of 3.61 (uncorrected p < .0005) was used for all of the following t-map analyses, as well as a volume threshold of 100 contiguous voxels at or above this t-threshold. Table 4 lists the results of the PET t-map analysis in which the pre-smoking RT baseline condition was subtracted from the dichotic listening conditions immediately prior and immediately following smoking of placebo and marijuana. Prior to smoking, the subtraction of the RT baseline from the dichotic condition replicated our previous findings (O'Leary et al. 1996a, 1997; Block et al. 2000), with dichotic listening resulting in large increases in rCBF in left (L) and right (R) superior temporal gyri (STG), and with no other regions showing significantly increased rCBF. Prior to smoking, there were only a few small regions with lower rCBF in the dichotic condition than in the RT baseline, which is also in line with our previous work. Smoking a placebo cigarette did not alter the pattern of activation in L and R STG for the dichotic minus baseline conditions. There were, however, additional small activations in L anterior temporal lobe, and R frontal lobe. Regions with lower rCBF in the dichotic than RT condition following placebo were found in L posterior cingulate, L superior parietal lobe, and L hippocampus. As discussed below, some of the rCBF differences between the pre-smoking condition and the condition following placebo appear to have been residual effects of marijuana since half of the subjects smoked marijuana prior to smoking placebo.
As seen in Table 4, smoking marijuana resulted in dramatic changes in rCBF when the pre-smoking baseline condition was subtracted from the dichotic condition immediately following smoking. In contrast to the pre-smoking and placebo analyses there were no significant activations in L or R STG. Extensive bilateral regions of ventral frontal and temporal lobes and insula had increased rCBF following smoking marijuana, as did the anterior cingulate and cerebellum. A number of regions also showed lower rCBF following smoking marijuana. These included several regions of L frontal lobe, L parietal lobe, L insula, and an extensive region of precuneus.
Table 5 and Figure 1 display the analyses directly subtracting the pre-smoking dichotic condition from the dichotic conditions immediately following smoking of a placebo and marijuana cigarette. These analyses allow comparison of the effects of smoking placebo versus marijuana on rCBF when subjects are performing the same cognitive task. Smoking a placebo cigarette resulted in relatively little change in rCBF from the pre-smoking condition (small increases in R and L frontal lobes and a small decrease in L parietal lobe). As in the RT baseline analysis, smoking marijuana resulted in increased rCBF in a number of regions in ventral and mesial frontal lobe, insula, temporal poles, and cerebellum. Smoking marijuana also resulted in decreased rCBF bilaterally in a number of regions in left and right frontal lobes, in L STG, and in R occipital lobe.
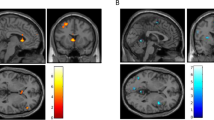
PET t-map image for the pre-smoking minus post-smoking marijuana analysis (in color) overlaid on an average MR image for the 12 subjects in the study. Subjects were performing the same dichotic task prior to and following smoking. The three columns on the right are axial, sagittal, and coronal views of the unthresholded t-map, with t values ranging from −6.0 (purple) to +6.0 (red) as illustrated on the pallet on the right. The three views in the column on the left represent the same t-map overlaid on the average MRI, but with the t values thresholded at a value of 3.61 (uncorrected p < .0005). Radiological convention is followed and the crosshairs are at the same location in all three views. The axial view shows a region in the anterior cingulate that has significantly higher t values (i.e., greater rCBF) after smoking marijuana, and regions in left and right superior temporal gyrus and occipital lobe that have lower t values (decreased rCBF) after smoking. The sagittal view again illustrates the increase in anterior cingulate rCBF and the decrease in rCBF in occipital lobe after smoking marijuana, and additionally illustrates a region in the inferior cerebellum that has higher rCBF following smoking. The coronal views illustrate bilateral regions of the temporal poles, insula and orbital frontal lobe that have significantly higher rCBF after smoking marijuana. Note that the regions of increased rCBF in the coronal view are lateral and ventral to the basal ganglia.
Table 6 presents the results of a direct comparison of the two post-smoking conditions. When the condition following smoking placebo was subtracted from the condition following smoking marijuana there were again regions of increased rCBF in L and R temporal poles, L ventral frontal lobe, R insula, R putamen, and in the cerebellum. Regions with lower rCBF following marijuana were observed in a number of bilateral frontal regions, in L STG and in R occipital lobe.
Because half of the subjects smoked marijuana prior to smoking placebo, the post-placebo condition may have been influenced by residual effects of marijuana, which was smoked approximately 50–60 min prior to smoking placebo. This would attenuate the effects of the marijuana versus placebo analysis because the post-placebo condition would also have marijuana effects for half of the subjects, which would be common to the two conditions and be subtracted out. To assess possible carryover effects separate analyses were performed for the two sets of six subjects who smoked placebo and marijuana in different orders.
For the analysis in which the pre-smoking baseline condition was subtracted from the condition that immediately followed smoking placebo, the placebo-first group had positive activations only in L and R STG. Therefore, the activations outside of STG following smoking placebo noted in Table 4 appear to be due to effects of smoking marijuana. Similarly, for the analysis in which the pre-smoking dichotic condition was subtracted from the condition immediately following smoking placebo, the placebo-first group had no significant regions of increased rCBF following smoking placebo. These analyses indicate that smoking placebo did not increase rCBF in any brain region. There was also no evidence that smoking placebo decreased rCBF in any brain region.
DISCUSSION
Smoking marijuana significantly increased HR and blood pressure, and resulted in extensive changes in rCBF in comparison to pre-smoking conditions, and to the conditions following smoking placebo. The rCBF changes we observed reflect the direct changes caused by smoking marijuana upon brain metabolism and blood flow, as well as less direct effects resulting from its intoxicating and mood-enhancing effects. Smoking marijuana increased rCBF in ventral forebrain regions that have extensive interconnections with the limbic system. As discussed below, these rCBF increases in “paralimbic” brain regions (Mesulam and Mufson 1982) had both similarities and differences to those reported in previous studies (Volkow et al. 1991a,b, 1996: Mathew et al. 1989, 1992, 1997), and may underlie the changes in affect that are frequently caused by smoking marijuana. Large regions of the cerebellum had rCBF increases, which have also been reported in previous studies, and may be associated with the intoxication caused by marijuana (Volkow et al. 1996).
Smoking marijuana also resulted in decreased rCBF in a number of brain regions, and altered the pattern of rCBF during the performance of an auditory attention task. Temporal lobe auditory regions that have consistently shown robust activation during the dichotic task in our laboratory (O'Leary et al. 1996, 1997; Block 2000), and in others’ (Hugdahl et al.1999, 2000), did not show rCBF increases that were significantly different from a baseline condition. Additionally, marijuana decreased rCBF in comparison to the baseline condition in brain regions that have been found in a number of studies to be involved in attentional modulation of sensory processing (Chelazzi and Corbetta 2000).
Whereas rCBF increases were localized to ventral forebrain and cerebellum, decreases in rCBF were localized to brain regions that mediate sensory processing and attention. These findings suggest that it may be possible to isolate the mood-enhancing effects of marijuana (rCBF increases in ventral forebrain) from marijuana's effects on perception, attention and behavior (decreased rCBF in sensory regions and attention-related brain systems).
Smoking Marijuana Increases Heartrate and Blood Pressure and Lowers Whole Brain PET Counts
Smoking marijuana increased HR by a mean of 40.6 beats/min in the PET condition immediately following smoking, with all 12 subjects showing this effect (increases ranged from 8 to 82 beats/min). The HR increase dropped slowly, remaining significantly elevated in the second (27.3 beats/min higher) and third (24.2 beats/min higher) PET conditions following smoking (about 35 and 50 min respectively after smoking). Diastolic blood pressure also increased after smoking marijuana, but the changes were smaller and more variable across individuals. Smoking marijuana did not result in a significant change in systolic blood pressure.
A recent review of the cardiovascular effects of marijuana noted that a dose-related tachycardia is a consistent finding in human studies, with increases in HR of 30–60% over control rates (Trouve and Nahas 1999). Trouve and Nahous note that marijuana consistently produces peripheral vasodilation in humans, but that the findings concerning blood pressure are less consistent. The most frequent finding is an increase in blood pressure (which is not seen in all subjects) when the subject is supine, but significant hypotension when the subject is upright.
In contrast to the increases found in humans, animal studies have consistently found evidence of decreased HR and blood pressure after injection of cannabinoids (for a review see Kunos et al. 2000a,b; Hillard 2000). It seems unlikely that the difference is due to the effects of smoking rather than injecting THC, since we found no significant cardiovascular effects in the present study when subjects smoked marijuana cigarettes with most of the THC removed. Animal studies have also found that injection of cannabinoids results in peripheral vasodilation. Endogenous lipidlike substances have been identified recently, (e.g., anandamide) that bind to cannabinoid receptors. It has been found that both plant-derived cannabinoids and the endogenous ligands produce decreased HR and hypotension in animals by activating CB1 receptors in the periphery (Kunos et al. 2000a,b). Anandamide has also been shown to cause vasodilation in mesenteric vascular beds that is independent of CB1 or CB2 receptors (Kunos et al. 2000b).
The results of the present study are consistent with previous human studies in finding that smoking marijuana increases HR dramatically, and causes less consistent increases in blood pressure. We also observed a significant drop in whole brain PET counts in the present study, which may be related to the cardiovascular effects of marijuana. [15O]water is a diffusible tracer which partitions into all tissues of the body in proportion to the blood flow to the tissue. Smoking marijuana caused significant increases in HR and blood pressure, and is likely to have induced peripheral vasodilation and changes in blood flow to other organ systems that resulted in lower amounts of radiotracer being available for deposition in the brain.
Although whole brain PET counts dropped, whole brain blood flow, measured quantitatively, was not significantly changed by smoking marijuana (see Table 2). The magnitude of regional radioactivity levels (i.e., counts/pixel), which sum to yield whole brain PET counts, is a function of both regional cerebral blood flow and the arterial input function. In previous studies we found that any manipulation that alters the arterial input function (e.g., changes in rate of injection, HR, skeletal muscle tone) alters the magnitude of the regional radioactivity levels totally independent of cerebral blood flow changes (Ponto et al. 1999, 2000). In the present study we found that the physiological changes caused by smoking marijuana altered the shape and magnitude of the arterial input function, and therefore altered delivery of tracer to the brain. But, as we have observed in previous studies, this was independent of brain blood flow changes measured quantitatively.
Behavioral Performance
Although smoking marijuana resulted in intoxication, and significantly changed rCBF during performance of the dichotic target detection task, behavioral performance was not significantly altered. The dichotic task was selected to provide a stable cognitive baseline state in order to assess the effects of marijuana unconfounded by changes in mental content resulting from intoxication. The task required spatially-selective attention (i.e., attention to the left ear) for the duration of each 170-s trial, as well as simple reaction time to 21 auditory target stimuli. A number of studies by our group and others have shown that smoking marijuana results in impairment on a variety of cognitive tasks (Block and Wittenborn 1984, 1986; see Solowij 1998 for a review). However, focused vigilant attention for durations less than 10 min, as required for the dichotic task, may not be impaired by marijuana (Vachon et al. 1974). Similarly, simple RT may not be impaired by smoking marijuana (Chait and Pierri 1992), although choice and complex RT tasks are more likely to result in impaired performance (reviewed in Solowij 1998). The fact that behavioral performance was not significantly changed after smoking marijuana in the present study indicates that the rCBF changes we observed were not a consequence of inattention or poor task performance.
Smoking Marijuana Increases rCBF in Paralimbic Brain Regions and Cerebellum
The rCBF increases after smoking marijuana (Tables 3–5) have both similarities and some interesting differences to previous PET studies. The rCBF increases replicated our initial study, which found increases in anterior cingulate, mesial and orbital frontal lobes, insula, temporal poles, and cerebellum (O'Leary et al. 2000). These findings are also largely in line with studies of the effects of injected THC. As noted in the beginning of this article, Volkow et al. (1996) found that both chronic marijuana users and control subjects had increased glucose metabolism in cerebellum, prefrontal, frontal, and right temporal cortices following injection of THC. Only chronic users had increases in orbital frontal cortex and basal ganglia. Using [15O]H2O, Mathew et al. (1997, 1998) found increased rCBF in R and L frontal lobes, temporal and parietal lobes, the cingulate gyrus, bilateral insula, basal ganglia and thalamus after injection of THC.
The anterior insula, lateral orbital frontal, and temporopolar regions that showed rCBF increases in our study are strongly interconnected, and may form a functional unit (Mesulam and Mufson 1982). Mesulam and Mufson suggested that these regions comprise a paralimbic area, functioning as an integrated unit that mediates interactions between extrapersonal space and the internal milieu. As such, increased rCBF in these regions may be the neurobiological basis of the changes in mood that are frequently induced by smoking marijuana. This possibility is supported by the fact that the orbital frontal activation appears to include the extended amygdala (Fudge and Haber 2001). Recent animal and human studies indicate that the amygdala may influence autonomic and hormonal changes, as well as overt motor behavior and attentional processes, in response to stimuli that have both positive and negative valence (Davis and Whalen 2001).
The anterior cingulate, which has strong ties to the limbic system, also had increased rCBF in our present and previous study (O'Leary et al. 2000). A recent meta-analysis of functional imaging studies found anterior cingulate activity to be most strongly associated with task difficulty (Paus et al. 1998). As noted above, performance on the dichotic target detection task did not change significantly after smoking marijuana, despite self-reports of intoxication. It seems possible that the increased rCBF in anterior cingulate following smoking may reflect the greater conscious effort required by the subjects to maintain the same level of performance on the task.
We found significantly increased rCBF in the cerebellum following smoking marijuana, with the effect largest on the right. This is in line with Volkow et al.’s (1996) finding of increased cerebellar metabolism following THC injection. Volkow et al. found that the cerebellar increase had a significant correlation with subjective ratings of intoxication. In contrast, Mathew et al. (1997) found that ratings of intoxication correlated significantly with frontal lobe rCBF in 32 volunteers. In an expansion of his sample to 46 volunteers, Mathew et al. (1998) found significantly increased rCBF in the cerebellum, but reported that not all subjects showed this effect. Subjects who had decreased cerebellar rCBF also had a disturbance of time sense. We plan to perform correlational analyses of ratings of intoxication with rCBF, using an approach that allows assessment of the correlation of a “seed voxel” or an external vector such as intoxication ratings with rCBF in every voxel of the PET image (Friston et al. 1993). Because this technique has relatively low power with small numbers of subjects, however, we plan to perform this analysis after the completion of a study that is currently underway, which will double the number of available subjects.
Smoking Marijuana Does Not Increase rCBF in Basal Ganglia, Nucleus Accumbens, and Hippocampus
The major discrepancy between our findings and those from other laboratories involve the basal ganglia. PET studies using injected THC have found significant increases in basal ganglia rCBF, whereas our data shows rCBF increases that are ventral and lateral to the basal ganglia. It is also possible that injected THC has different effects on brain metabolism and blood flow than does smoked marijuana. However, another explanation for the divergent findings is that the studies by Volkow et al. (1996) and Mathew et al. (1992) utilized ROIs based upon predefined templates that averaged activity over relatively large volumes of tissue. This could have resulted in activations near to, but outside of, basal ganglia being averaged into basal ganglia ROIs.
The present study utilized individually co-registered PET and high-resolution MR images that permitted more precise anatomical localization than in previous studies. AIR software (Woods et al. 1992) was used to register each of the eight PET conditions for each individual to their MR image. Landmarks on the MRI were then used to place all images into a standardized stereotaxic atlas space (Talairach and Tournoux 1988), which allowed averaging of both PET and MR image sets. The lenticular nuclei can be visualized on the average MRI, and, as seen in Figure 1, significant PET activations are ventral and lateral to these structures. Although the nucleus accumbens is a small structure that is difficult to visualize with MRI, its general location can be found on the average MRI. Significant areas of activation were lateral to this, suggesting that marijuana did not increase rCBF significantly in “reward-related” brain regions.
The hippocampi as well as the basal ganglia contain a high density of cannabinoid receptors, and also failed to show significant rCBF changes following smoking of marijuana. Inspection of the PET t-map images in the mesial temporal lobe, aided by the co-registered MR image, showed no region close to the hippocamal formation with significantly increased or decreased rCBF. This lack of rCBF change in brain regions with high densities of cannabinoid receptors indicates that the large blood flow changes observed in the present study result from increased synaptic activity downstream from the receptor binding sites. Our failure to find rCBF changes in brain regions rich in cannabinoid receptors (except for cerebellum) indicates that the immediate metabolic effects of THC binding to cannabinoid receptors are relatively brief.
It remains controversial whether marijuana is an atypical or anomalous addictive drug, which interacts with brain reward systems differently than drugs such as methamphetamine that directly activate brain DA systems (e.g., Gardner and Vorel 1998). Our finding of no rCBF changes in the nucleus accumbens and basal ganglia may be taken as support for the position that smoking marijuana does not directly activate reward-relevant DA neurons in the nucleus accumbens and/or basal ganglia. However, because the subjects had to be repositioned in the PET camera after smoking, our rCBF measures occurred approximately 15 min after smoking marijuana, which may have been too late to observe the direct effects of THC binding to receptors. It is also possible that our technique, which assessed rCBF changes using subtraction analysis and statistical mapping, may not have been sensitive to subtle changes in metabolism in reward-related brain regions.
Smoking Marijuana Decreases rCBF in Sensory Cortices and in Attention-Related Brain Regions
Smoking marijuana did not significantly change performance on the dichotic listening task but did result in decreased rCBF in auditory processing regions of the temporal lobes that had been activated by the dichotic task prior to smoking. As can be seen in Table 4, subjects performing the dichotic listening task had lower rCBF in Heschl's gyrus in the temporal lobe after smoking marijuana than they did prior to smoking. It is important to note that smoking marijuana did not result in decreased rCBF in Heschl's gyrus in comparison to the pre-smoking baseline condition (see Table 3). Rather, the effect of marijuana was to decrease the magnitude and extent of the activation resulting from the dichotic task. The regions showing decreased rCBF contains both primary and secondary auditory cortex (Liegeois-Chauvel, Musolino and Chauvel 1991). Thus the effect of smoking marijuana was to eliminate the task-related activation of auditory processing regions that is normally caused by the dichotic task.
In previous studies of dichotic listening we have found that activation in STG reflects attentional processing in normal volunteers, and we have found abnormal activation patterns in STG in subject populations that may have attentional impairment (see O'Leary, in press for a review). In normal volunteers, attention to the right ear increased the spatial extent and magnitude of rCBF to a greater degree in left STG, which was contralateral to the direction of attention, than in right STG (O'Leary et al. 1996a). Attending to the left ear reversed this asymmetry, with greater rCBF increases in right than left STG. This finding has been replicated in normal volunteers in two other studies performed by our group (Hurtig et al. unpublished manuscript; Block et al. 2000). In the present study we found that both left and right STG showed significant activation in the pre-smoking baseline minus pre-smoking dichotic analysis (see Table 2). In contrast to our previous studies using an attend-left condition, left STG had a larger t-max (t = 10.0 vs. 9.0) and greater volume (16.7 vs. 11.6 cc) of activation than the right STG. The activation observed in both left and right STG due to the pre-smoking dichotic listening task in the present study was larger than in previous studies, and differences in the characteristics of this subject sample may explain the differences in rCBF asymmetry. We were not able to observe changes in rCBF asymmetry due to the direction of attention because attend right conditions were not included in the present study. This comparison is included in a companion study currently underway in our laboratory.
A group of individuals with schizophrenia assessed in a previous study had similar rCBF patterns in STG to a volunteer group when attending to the right ear, but failed to show the normal change in rCBF asymmetry when attending to the left ear (O'Leary et al. 1996b). That is, the patient group maintained a left greater than right asymmetry regardless of where attention was directed. We recently found that chronic users of marijuana (minimum use of more than 7 times weekly for more than 2 years) also had an abnormal pattern of asymmetry when attending to the left ear (Block et al. 2000). The subjects were tested after 26 h of monitored abstinence using the same baseline dichotic stimuli used in the present study, but with both attend left and attend right conditions. The chronic marijuana users resembled schizophrenics in showing greater left than right STG activation when attention was directed to either the left or right ears. The abnormal pattern of activation observed in both populations may reflect an inability to voluntarily activate the right hemisphere during the more difficult attend-left condition and to thereby reverse the normal left hemisphere advantage for linguistic stimuli.
As can be seen in Table 4, smoking marijuana decreased rCBF bilaterally in the precentral sulcas/gyrus (i.e. motor strip), and in left parietal lobe in the vicinity of the intraparietal sulcas. Chelazzi and Corbetta (2000) recently reviewed PET imaging studies in which subjects covertly directed attention to visual stimuli in peripheral locations. Regions that were found to show consistent attention-related activation included the precentral sulcus/gyrus, and areas of parietal cortex (the postcentral and intraparietal sulcus) that showed decreased blood flow in the present study. Bushara et al. (1999) assessed the frontal and parietal regions that were activated during auditory and visual spatial localization tasks. They found regions in the frontal and parietal lobes that were uniquely activated during auditory and visual tasks as well as “supra-modal” regions that responded to both modalities. A previous study by our group (O'Leary et al. 1997) contrasted attention to auditory and to visual stimuli. A subtraction of a resting baseline condition from averaged attend left and attend right dichotic conditions revealed activations in right parietal lobe (BA 7), left frontal lobe (BA 4), right insula, and right cerebellum, but the network activated by auditory attention was much less extensive than that activated by visual attention. Thus, smoking marijuana decreased rCBF in frontal and parietal regions that have been found to play a role in the attentional enhancement of sensory processing, which may explain the lower rCBF observed in auditory cortices in the dichotic conditions following smoking.
References
Andreasen NC, Cizadlo T, Harris G, Swayze V, O'Leary DS, Cohen G, Ehrhardt J, Yuh WTC . (1993): Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci 5: 121–130
Biegon A, Kerman I . (1995): Quantitiative autoradiography of cannabinoid receptors in the human brain post mortem. In Biegon A, Volkow (eds), Sites of Drug Action in the Human Brain. Boca Raton, FL, CRC Press, pp 65–74
Block RI, Farinpour R, Braverman K . (1992): Effects of marijuana smoking on cognition and their relationship to smoking technique. Pharmacology Biochemistry & Behavior 43: 907–917
Block RI, Wittenborn JR . (1984): Marijuana effects on semantic memory: verification of common and uncommon category members. Psychol Rep 55: 503–512
Block RI, O'Leary DS, Augustinack JC, Boles Ponto LL, Ghoneim MM, Hurtig RR, Hall JA, Nathan PE . (2000): Effects of frequent marijuana use on attention-related regional cerebral blood flow. Society for Society for Neuroscience Abstracts 26 (Part 2): 2080
Block RI, Wittenborn JR . (1986): Marijuana effects on speed of memory retrieval on a letter matching task. Int J Addict 21: 281–285
Bushara KO, Weeks RA, Ishii K, Catalan MJ, Tian B, Rauschecker JP, Hallett M . (1999): Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci 2: 759–766
Chait LD, Pierri J . (1992): Effects of smoked marijuana on human performance: a critical review. In Murphy L, Bartke A (eds), Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, CRC Press, pp 387–423
Chelazzi L, Corbetta M . (2000): Cortical mechanisms of visuospatial attention in the primate brain. In M Gazziniga (ed), The New Cognitive Neurosciences. 2nd ed. Cambridge, MA, MIT Press, pp 648–663
Cizadlo T, Andreasen NC, Zeien G, Rajarethinam R, Harris G, O'Leary DS, Swayze VW, Arndt S, Hichwa R, Ehrhardt J, Yuh WTC . (1994): Image registration issues in the analysis of multiple-injection 150H20 PET studies: BRAINFIT. Proceedings from SPIE-The International Society for Optical Engineering 2168: 234–245
Davis M, Whalen PJ . (2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6: 13–34
Friston KJ, Frith CD, Frackowiak RSJ . (1993). Time-dependent changes in effective connectivity measured with PET. Human Brain Mapping 1: 69–79
Fudge HL, Haber JN . (2001): Bed nucleus of the stria terminalis and extended amygdala inputs to dopamine subpopulations in primates. Neuroscience 104: 807–827
Gardner EL, Vorel SR . (1998): Cannabinoid transmission and reward-related events. Neurobiol Dis 5: 502–533
Harvey DJ . (1999): Absorption, distribution and biotransformation of the cannabinoids. In Nahas GG, Sutin KM, Harvey DJ, Agurell S (eds), Marijuana and Medicine. Totawa NJ, Humana Press, pp 91–103
Heishman SJ, Huertis MA, Henningfield JE, Cone EJ . (1990): Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacology Biochemistry and Behavior 37: 561–565
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC . (1990): Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences 87: 1932–1936
Herkenham M . (1992): Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann NY Acad Sci 654: 19–32
Herscovitch P, Markham J, Raichle ME . (1983): Brain blood flow measured with intravenous H215O. I. Theory and error analysis. Journal of Nuclear Medicine 24: 782–789
Hichwa RD, Ponto LLB, Watkins GL . (1995): Clinical blood flow measurements with [15O]water and positron emission tomography (PET). In Emran AM (ed), Symposium Proceedings of the International Symposium on “Chemists’ Views on Imaging Centers.”. New York, Plenum, pp 401–417
Hillard CJ . (2000): Endocannabinoids and vascular function. J Pharmacol Exp Ther 294: 27–32
Huestis M . (1999): Pharmacokinetics of THC in inhaled and oral preparations. In Nahas GG, Sutin KM, Harvey DJ, Agurell S (eds), Marijuana and Medicine, Totawa, NJ, Humana Press pp 105–116
Hugdahl K, Bronnick K, Kyllingsbaek S, Law I, Gade A, Paulsen OB . (1999): Brain activation during dichotic presentations of consonant-vowel and musical instrument stimuli: A 15O-PET study. Neuropsychologia 37: 431–440
Hugdahl K, Law I, Kyllingsbaek S, Bronnick K, Gade A, Paulson OB . (2000): Effects of attention on dichotic listening: An 15O-PET study. Hum Brain Mapp 10: 87–97
Hurtig RR, Hichwa RD, O'Leary DS, Boles Ponto LL, Narayana S, Watkins GL, Andreasen NC . (1994): Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab 14: 423–430
Johnston LD, O'Malley PM, Bachman JG . (1999): Drug trends in 1999 are mixed. Monitoring the Future Study. University of Michigan News and Information Services
Kunos G, Jarai Z, Batkai S, Goparaju SK, Ishac EJ, Liu J, Wang L, Wagner JA . (2000a): Endocannabinoids as cardiovascular modulators. Chem Phys Lipids 108: 159–168
Kunos G, Jarai Z, Varga K, Liu J, Wang L, Wagner JA . (2000b): Cardiovascular effects of endocannabinoids–the plot thickens. Prostaglandins Other Lipid Mediat 61: 71–84
Liegeois-Chauvel C, Musolino A, Chauvel P . (1991): Localization of the primary auditory area in man. Brain 114: 139–153
Loeber RT, Yurgelun-Todd DA . (1999): Human neuroimaging of acute and chronic marijuana use: implications for frontocerebellar dysfunction. Human Psychopharmacology ClinExp 14: 291–304
Mathew RJ, Wilson WH, Tant SR . (1989): Acute changes in cerebral blood flow associated with marijuana smoking. Acta Psychiatr Scand 79: 118–128
Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE . (1992): Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab 12: 750–758
Mathew RJ, Wilson WH, Coleman RE, Turkington TG, DeGrado TR . (1997): Marijuana intoxication and brain activation in marijuana smokers. Life Sci 60: 2075–2089
Mathew RJ, Wilson WH, Turkington TG, Coleman RE . (1998): Cerebellar activity and disturbed time sense after THC. Brain Res 797 (2): 183–189
Mesulam M-M, Mufson EJ . (1982): Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurolog 212: 38–52
O'Leary DS . Effects of attention on hemispheric asymmetry. In Hugdahl K, Davidson R (eds), Brain Asymmetry, Cambridge, MA, MIT Press (in press)
O'Leary DS, Andreasen NC, Hurtig RR, Hichwa RD, Watkins GL, Boles Ponto LL, Rogers M, Kirchner PT . (1996a): A regional cerebral blood flow study of language and auditory attention. Brain Lang 53: 20–39
O'Leary DS, Andreasen NC, Hurtig RR, Kesler ML, Rogers M, Arndt S, Cizadlo T, Watkins GL, Ponto LLB, Kirchner PT, Hichwa RD . (1996b): Auditory attentinal deficits in patients with schizophrenia: a positron emission tomography (PET) sutdy. Arc Gen Psychiatry 53: 633–641
O'Leary DS, Andreasen NC, Hurtig RR, Torres IJ, Flashman LA, Kesler ML, Ponto LLB, Watkins GL, Hichwa RD . (1997): Auditory and visual attention assessed with PET. Hum Brain Mapp 5: 422–436
O'Leary DS, Block RI, Flaum M, Schultz SK, Boles Ponto LL, Watkins GL, Hurtig RR, Andreasen NC, Hichwa RD . (2000): Acute marijuana effects on rCBF and cognition: A PET study. Neuroreport 11: 1–7
Paus T, Koski L, Caramanos Z, Westbury C . (1998): Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 9: R37–47
Pertwee RG . (1997): Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74: 129–180
Ponto LLB, Popowski LA, Gisolfi CV, Johnson AK, Madsen MT, Watkins GL, Hichwa RD, Bushnell D . (1999): Influence of hydration state and cognitive performance on the CNS deposition of freely-diffusible substances. AAPS PharmSci (supplement) 1: S-123
Ponto LLB, Narayana S, Grabowski TJ . (2000): Pharmacokinetic examination of the interaction between skeletal muscle blood flow and CNS deposition of freely-diffusible substances. AAPSPharmSci [serial on the internet]. 2 (4): electronic. SAMHSA, National Household Survey on Drug Abuse (1998, 1999).
SAMHSA. (1998, 1999): National Household Survey on Drug Abuse, Rockville, MD, Author
Solowij N . (1998): Cannabis and Cognitive Functioning, Cambridge, Cambridge University Press
Talairach J, Tournoux P . (1988): Co-planar Stereotaxic Atlas of the Human Brain 3-D Proportional System: An Approach to Cerebral Imaging. New York, Thieme
Trouve R, Nahas G . (1999): Caardiovascular effects of marijuana and cannabinoids. In Nahas GG, Sutin KM, Harvey DJ, Agurel S (eds), Marijuana and Medicine. Totowa, NJ, Humana Press, pp 291–304
Vachon L, Sulkowski A, Rich E . (1974): Marijuana effects on learning, attention, and time estimation. Psychopharmacologicia 39: 1–11
Volkow ND, Gillespie H, Mullani M, Tancredi L, Hollister L, Ivanovic M, Grant C . (1991a): Use of positron emission tomography to investigate the action of marijuana in the human brain. Adv Biosci 80: 3–11
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Ivanovic M, Hollister L . (1991b): Cerebellar metabolic activation by delta-9-tetrahydrocannabinol in human brain: A study with positron emission tomography and 18F–2-fluorof-2-deoxyglucose. Psychiatry Research. Neuroimaging 40: 69–78
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L . (1996): Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Research: Neuroimaging 67: 29–38
Wollenweber SD, Hichwa RD, Ponto LLB . (1997): A simple on-line arterial time-activity curve detector for [O-15]water studies (1997). IEEE Trans Nuclear Science 44: 1613–1617
Woods RP, Cherry SR, Mazziotta JC . (1992): Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 16: 620–633
Worsley K, Evans A, Marret S, Neelin P . (1992): A three dimensional statistical analysis for CBF activation studies in the human brain. J Cereb Blood Flow Metab 12: 900–918
Acknowledgements
This research was supported in part by the National Institute of Drug Abuse Grant DA10551 and by MHCRC43271.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O'Leary, D., Block, R., Koeppel, J. et al. Effects of Smoking Marijuana on Brain Perfusion and Cognition. Neuropsychopharmacol 26, 802–816 (2002). https://doi.org/10.1016/S0893-133X(01)00425-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00425-0
Keywords
This article is cited by
-
Biological activity of Cannabis compounds: a modern approach to the therapy of multiple diseases
Phytochemistry Reviews (2022)
-
The Cerebellum, THC, and Cannabis Addiction: Findings from Animal and Human Studies
The Cerebellum (2019)
-
Residual Effects of THC via Novel Measures of Brain Perfusion and Metabolism in a Large Group of Chronic Cannabis Users
Neuropsychopharmacology (2018)
-
Altered Gray Matter Volume in Stable Chronic Obstructive Pulmonary Disease with Subclinical Cognitive Impairment: an Exploratory Study
Neurotoxicity Research (2017)
-
Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses
Biology of Mood & Anxiety Disorders (2013)