Abstract
Reprogramming of somatic cells in the enucleated egg made Dolly, the sheep, the first successfully cloned mammal in 1996. However, the mechanism of sheep somatic cell reprogramming has not yet been addressed. Moreover, sheep embryonic stem (ES) cells are still not available, which limits the generation of precise gene-modified sheep. In this study, we report that sheep somatic cells can be directly reprogrammed to induced pluripotent stem (iPS) cells using defined factors (Oct4, Sox2, c-Myc, Klf4, Nanog, Lin28, SV40 large T and hTERT). Our observations indicated that somatic cells from sheep are more difficult to reprogram than somatic cells from other species, in which iPS cells have been reported. We demonstrated that sheep iPS cells express ES cell markers, including alkaline phosphatase, Oct4, Nanog, Sox2, Rex1, stage-specific embryonic antigen-1, TRA-1-60, TRA-1-81 and E-cadherin. Sheep iPS cells exhibited normal karyotypes and were able to differentiate into all three germ layers both in vitro and in teratomas. Our study may help to reveal the mechanism of somatic cell reprogramming in sheep and provide a platform to explore the culture conditions for sheep ES cells. Moreover, sheep iPS cells may be directly used to generate precise gene-modified sheep.
Similar content being viewed by others
Introduction
Sheep have been an important domesticated animal for a long time. Dolly, the sheep, was the first cloned mammal generated by somatic cell nuclear transfer (SCNT) in 1996 1. Gene modification in sheep to improve their production traits, disease resistance ability, and ability to produce valuable, high-quality proteins has been widely applied in agriculture and biomedicine 2, 3, 4. However, traditional transgenic techniques result in the poor control of exogenous genes and extremely low efficiency, which greatly limits the application of gene-modified sheep.
The gene targeting technique based on embryonic stem (ES) cells, which has been well established in mouse 5, 6, 7, allows precise and effective gene knockin and knockout. To date, ES cells have been isolated from a few species, including mouse 5, 8, human 9, monkey 10 and rat 11, 12. However, it is difficult to isolate and maintain ES cells from sheep embryos and other ungulates, because little is known of the characteristics and culture conditions of the ESCs from these species. Therefore, although many efforts have been made to derive sheep ES cells during the last few decades, no authentic sheep ES cell line has been reported. Although SCNT has been widely used in sheep propagation and the generation of transgenic sheep, the mechanism of somatic cell reprogramming in sheep remains unclear.
Somatic cells can be reprogrammed using defined factors to achieve a pluripotent status 13, 14, 15. More importantly, induced pluripotent stem (iPS) cells have been demonstrated to efficiently contribute to the germline to allow precise genetic engineering in vivo 16, which means that iPS cells could be used as a substitute for ESCs to generate transgenic animals. In recent years, iPS cells have been generated from many species, including mouse 14, 17, human 13, 15, rat 18, 19 and pig 20, 21, 22. In this study, we reprogrammed somatic cells from sheep to pluripotency using defined factors.
Results
Generating sheep iPS cell lines using a Tet-On inducible lentiviral system
We initially tried to use a constitutively expressed lentivirus vector carrying OSCK (Oct4, Sox2, c-Myc and Klf4) or OSCKNL (Oct4, Sox2, c-Myc, Klf4, Nanog and Lin28) to reprogram sheep fibroblasts. The sheep primary fibroblasts were isolated from the ears of a 4-week-old sheep, and were expanded for a few passages before virus transduction. We established a few ES-like cell lines exhibiting ES cell characteristics, including round, tightly packed morphology, alkaline phosphatase (AP) activity, expression of ES cell markers and the ability to differentiate into three germ layers in vitro. The reprogramming efficiency was 10 AP-positive colonies from 1 × 105 fibroblasts. However, these cells could not be stably expanded while maintaining an undifferentiated state. Moreover, these cells failed to differentiate into the three germ layers in teratomas, probably because of the constitutive expression of exogenous genes (data not shown).
To solve this problem, we tried to reprogram sheep fibroblasts with a drug-inducible lentivirus system. The doxycycline (DOX)-inducible transgene expression vectors were constructed, in which Oct4, Sox2, c-Myc, Klf4, Nanog and Lin28 were inserted downstream of a tetracycline operator. The expression of reverse tetracycline transactivator (rtTA) was driven by the EF-1 α promoter in a separate lentiviral vector. The DOX-inducible system has been shown to work very well 20. The cocktail of lentiviruses was used to transduce sheep fibroblasts. However, the efficiency of generating AP-positive colonies was extremely low (about one AP-positive colony from 1 × 105 fibroblasts). Moreover, these AP-positive colonies could not be stably expanded. Compared with the constitutively expression system, reprogramming sheep somatic cells using the DOX-inducible system was much more difficult, probably because of the low expression levels of exogenous genes. We speculate that the traditional six transcription factors were not sufficient to reprogram sheep adult cells with the DOX-inducible lentiviral system.
The SV40 large T antigen (T) and the catalytic subunit of human telomerase, hTERT, have been reported to significantly improve the efficiency of generating iPS cells 23, 24. Therefore, we attempted to establish ovine iPS cell lines by adding SV40 large T and hTERT.
The fibroblasts were simultaneously transduced with a cocktail of lentiviruses expressing reprogramming factors (Oct4, Sox2, c-Myc, Klf4, Nanog, Lin28, SV40 large T and hTERT) and a lentivirus constitutively expressing rtTA. At 24 h after transduction, the cells were trypsinized and plated onto murine embryonic fibroblasts (MEFs) at 2.5 × 104 cells per well in a six-well plate. The next day, the original medium (Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS)) was replaced with medium for ES cell culture plus DOX (DMEM/F12 + 20% SR + 1 μg/ml DOX).
At 5 days after transduction, infected fibroblasts exhibited changes in morphology and began to proliferate very rapidly. On days 11 and 17, the cells were trypsinized into a single-cell suspension and passaged. We first observed large and round ESC-like colonies with clear boundaries (Figure 1C) on day 20. At 3 days later, we picked the colonies from half of the 6-well plates into a 96-well plate for further expansion. The remaining plates were subjected to AP staining to calculate the efficiency of colony generation. The sheep ESC-like cells (named sheep iPS cells or oiPSCs) were expanded on MEF feeder cells with ES cell medium plus DOX (1 μg/ml).
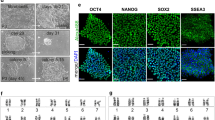
Ovine-induced pluripotent stem cells (oiPSCs) generated by viral transduction. (A) Schematic diagram of the reprogramming protocol used. (B) Typical non-embryonic stem (ES) cell-like colony. (C) Typical oiPSC colony. (D) High magnification of the oiPSCs. These iPSCs express the following pluripotency markers: (E) alkaline phosphatase (AP), (F) SSEA-1, (I) TRA-1-60, (J) TRA-1-81, (K) REX1 and (L) E-cadherin. oiPSCs do not express the human ES cell-specific surface antigen SSEA-3 (G) or SSEA-4 (H). The images (F-L) of immunostaining were acquired by confocal system. Scale bars: 100 μm in B, C and E; 25 μm in D and F-L.
In our study, we obtained sheep iPS cell lines with the combination of OSCKNL+T+hTERT, but failed when using the combination of OSCK+T+hTERT. About 41 AP-positive colonies were derived from 1 × 105 initial fibroblasts. To examine whether SV40 large T or hTERT was necessary, the two factors were withdrawn separately. Sheep iPS cells were successfully generated with the combination of OSCKNL+T, but not with the combination of OSCKNL+hTERT. Compared with mouse, human, rat and pig, whose iPS cells have been generated only with OSCK, the reprogramming of sheep somatic cells required additional factors when using the DOX-inducible lentiviral system. The addition of SV40 large T was especially important. Moreover, the efficiency of generating sheep iPS cells using all eight factors (OSCKNL+T+hTERT) was threefold lower than the efficiency of generating pig iPS cells using four factors (OSCK) with a Tet-On lentiviral system (about 170 AP-positive colonies from 1 × 105 fibroblasts 20). These results indicate that sheep somatic cells are more difficult to reprogram than somatic cells from other species.
Sheep iPS cells exhibit typical ES cell morphology and marker expression
OiPS cells exhibited a round, tightly packed morphology characterized by a high ratio of nucleus to cytoplasm and prominent nucleoli (Figure 1D), which is quite similar to mouse and rat ESCs, but not human ESCs. Two oiPS cell lines we established, oiPS8-108 and oiPS8-119, have already been stably passaged for more than 30 generations (more than 90 days). The doubling time of oiPSCs was about 17.33 h for oiPS8-108 and 16.43 h for oiPS8-119, and the plating efficiency was 28.4% ± 0.17% for oiPS8-108 and 41% ± 0.57% for oiPS8–119.
The oiPS cells exhibited AP activity (Figure 1E) and expressed ES cell makers, including OCT4, SOX2, NANOG (Supplementary information, Figure S1), stage-specific embryonic antigen (SSEA)-1 (Figure 1F), TRA-1-60 (Figure 1I), TRA-1-81 (Figure 1J), REX1 (Figure 1K) and E-cadherin (CDH1; Figure 1L). The cells showed an absence of the human ESC-specific surface makers, SSEA-3 (Figure 1G) and SSEA-4 (Figure 1H). The expression of E-cadherin suggests that the oiPSCs are reprogrammed beyond the state of 'near pluripotency' (the state of so-called FAB-SCs) to the state of 'full pluripotency' (the state of ES cells) 25, 26, 27. Moreover, RT-polymerase chain reaction (RT-PCR) showed that oiPSCs express many ES cell marker genes, including Oct4, Sox2, Nanog, Tdgf1, Eras, Dnmt3b, Dax1, Dppa4 and Gdf3 (Figure 2A).
(A) Reverse transcription-polymerase chain reaction (RT-PCR) analysis of the oiPSC lines compared with the gene expression observed in parental sheep primary cells. Abbreviation: -RT, minus reverse transcriptase. (B) Quantitative reverse transcription-PCR analyses of endogenous Oct4, Sox2 and Nanog expression in oiPSCs relative to parental somatic cell populations. oiPS7-3 and 7-12 represent sheep iPS cell lines generated with seven factors (OSCKNL+T) and oiPS8-3, 8-18, 8-60, 8-64, 8-108, 8-119 represent sheep iPS cell lines generated with eight factors (OSCKNL+T+hTERT).
We analyzed the endogenous expression of the pluripotent genes Oct4, Sox2 and Nanog by quantitative PCR and found that all three were robustly induced (Figure 2B).
Epigenetic status of sheep iPS cells
The epigenetic status of sheep iPSCs was evaluated using bisulfite genomic sequencing. We analyzed the promoter region of Nanog and found that the CpGs were highly unmethylated in sheep iPSCs compared to the parental fibroblasts (Figure 3A). These results indicate that the Nanog promoter was reactivated in sheep iPSCs, which is consistent with the robust induction of endogenous Nanog during reprogramming.
We also analyzed the karyotype of oiPSCs after passage 15, and the results showed that the sheep iPS clones exhibited a normal karyotype of 54 XY (Figure 3B).
The transgenes were highly expressed in oiPSCs. When DOX was withdrawn, the exogenous genes were markedly downregulated (Figure 3C), and the iPS colonies could not retain normal morphology and consequently underwent differentiation. This suggests that the sheep iPS cell lines we established could not maintain pluripotency in the current culture conditions without DOX.
Sheep iPSCs are able to differentiate into all three germ layers in vitro and in vivo
ES cells are able to differentiate into all three primary germ layers both in vitro and in vivo, which is a very important criterion of pluripotency. To test the differentiation capability of oiPSCs in vitro, we allowed the cells to form embryoid bodies and undergo differentiation in medium for 7 days, and then analyzed the presence of markers characterizing each germ layer. RT-PCR results showed that sheep iPS cells were able to differentiate into all the three germ layers, as evidenced by the high expression of Afp (endoderm), Dcn (endoderm), Foxa2 (endoderm), Fibronectin (ectoderm), Neurod (ectoderm), Nfh (ectoderm), Actc1 (mesoderm), Kdr (mesoderm), Myf5 (mesoderm) and Renin (mesoderm; Figure 4A).
(A) Reverse transcriptase-polymerase chain reaction analyses of various differentiation markers for the three germ layers in the embryoid body. Abbreviation: EB, embryoid body; −RT, minus reverse transcriptase. (B) Immunocytochemistry of SOX17, smooth muscle actin (SMA) and βIII-tubulin (TUJ-1). Scale bars: 50 μm.
We then examined the in vitro differentiation ability of sheep iPS cells by immunocytochemistry. Sheep iPS cells formed EBs and were transferred onto gelatin-coated plates for continued cultivation. Attached cells exhibited various types of morphologies. Immunocytochemistry detected cells positive for SOX17 (endoderm), smooth muscle actin (SMA; mesoderm) and βIII-tubulin (TUJ-1; ectoderm; Figure 4B).
To examine their pluripotency in vivo, we injected sheep iPS cells intramuscularly into non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. At 4 weeks after injection, tumors became visible. Histological examination showed that the tumors contained various tissues, including neural rosettes (ectoderm), squamous epithelia (ectoderm), bone (mesoderm), gut-like epithelial tissues (endoderm) and glands (endoderm; Figure 5). Thus far, we have observed the complete differentiation ability only of sheep iPS cells generated with all eight factors (OSCKNL+T+hTERT) in the teratomas. For unknown reasons, when the cell lines generated with seven factors (OSCKNL+T) were tested, no differentiation was observed in the teratomas.
Hematoxylin-eosin staining of teratomas derived from sheep-induced pluripotent stem cells. The types of tissues that can be found in the teratomas include (A) neural rosettes (ectoderm), (B) squamous epithelium (ectoderm), (C) bones (mesoderm), (D) gut-like epithelial tissue (endoderm) and (E) glands (endoderm). Scale bars: 50 μm.
Discussion
In this study, we established sheep iPS cell lines with a drug-inducible system to induce the expression of eight defined factors. The sheep iPSCs exhibited ES cell-specific characteristics, including characteristic morphology, AP activity, expression of various ES cell markers and the capability to differentiate into all three germ layers both in vitro and in vivo.
Sheep are an important domesticated species. Pluripotent stem cells, which are essential to generate precise gene-modified animals, have not yet been reported in sheep. In this study, we generated pluripotent stem cells from sheep by using iPS technology. Sheep iPS cells resemble mouse ES cells, rather than human ES cells, in morphology under our current culture conditions. These cells express SSEA-1, rather than SSEA-3 or -4. The properties of sheep iPS cells we revealed should be informative for the identification of sheep pluripotent stem cells and may greatly facilitate the establishment of authentic sheep ES cells.
Sheep iPS cells, whose properties are similar to those of ES cells, provide a unique platform to screen for essential growth factors and/or chemicals that help to maintain self-renewal and pluripotency of sheep ES cells. The most critical problem that has hindered the establishment of sheep ES cells is how to inhibit the differentiation of ES cells. Each of the existing ES cells, including human, monkey, mouse and rat ES cells, requires different culture conditions. It is reasonable to speculate that sheep ES cells need to be maintained under certain unknown culture conditions. Therefore, ES cell culture condition screening based on the sheep iPS cells should be an efficient approach to facilitate the eventual establishment of sheep ES cell lines.
Mouse iPS cells have been shown to be successful in homologous recombination 16 and the generation of chimeric 14, 17, 28 and clonal mice 29, 30. Sheep iPS cells may allow genetic manipulation by homologous recombination, and may be used as the nuclear donor to generate genetically modified sheep through SCNT.
Using a DOX-inducible lentivirus system, we have generated human (unpublished data) and pig iPS cells with either OSCK (Oct4, Sox2, c-Myc and Klf4) or OSCKNL (Oct4, Sox2, c-Myc, Klf4, Nanog and Lin28) combinations. However, we could not obtain sheep iPS cells with the same procedure, suggesting that somatic cells from sheep are more difficult to reprogram than somatic cells from human or pig.
We observed that adding SV40 large T and hTERT to the OSCKNL combination significantly increased the efficiency of sheep iPS cell production. Moreover, without SV40 large T, we were not able to obtain stable iPS cells from sheep somatic cells by overexpressing six factors (OSCKNL) or seven factors (OSCKNL+hTERT) with the DOX-inducible system. These observations suggest that SV40 large T plays an essential role in reprogramming sheep somatic cells. SV40 large T is an essential protein for SV40 DNA replication and disables the retinoblastoma and p53 tumor suppressor pathways 31, 32, 33, 34. It has been reported that knocking down p53 greatly increases reprogramming efficiency 35, 36, 37, 38, 39. We speculate that SV40 large T probably works by inhibiting p53 to increase reprogramming efficiency. Alternatively, SV40 large T may turn on certain genes or signaling pathways that are critical for sheep pluripotent stem cells or may cause reprogramming-related chromosome remodeling. hTERT, which has potent anti-apoptotic activity 40, may promote the generation and maintenance of sheep iPS cells by increasing cell proliferation and inhibiting cell death.
Trypsinization and passaging of transduced sheep fibroblasts during the reprogramming progress were critical. Since adding SV40 large T and hTERT increased the proliferation of cells, the transduced cells quickly formed very large colonies and became confluent. Therefore, we trypsinized the colonies into single cells, and passaged them onto new dishes to release the well-reprogrammed cells. By being trypsinized and passaged once or twice, the AP-positive colonies appeared about 20 days after viral transduction. Without trypsinizing and passaging, no ESC-like colonies could be obtained.
In the future, sheep iPS cells may be used to study the mechanisms of reprogramming and maintenance of pluripotency. These cells may also be used to generate precise gene-modified sheep by SCNT.
Materials and Methods
Cell culture
The sheep primary fibroblasts were derived from the ear of a young sheep and were cultured in α-DMEM culture medium (Invitrogen) supplemented with 10% FBS (Hyclone). The sheep iPSCs were maintained on irradiated CF-1 MEFs in DMEM/F12 supplemented with 20% KnockOut serum replacer, 0.1 mM non-essential amino acids, 1 mM L-glutamine and 0.1 mM β-mercaptoethanol (ES medium; all from Invitrogen, Carlsbad, CA, USA). The medium was also supplemented with 1 μg/ml DOX (Chemicon). The sheep iPSCs were split with TrypLE (Invitrogen) at a ratio of 1:10 every 3 days. To form embryoid bodies, the sheep iPSCs were dissociated with TrypLE and transferred to a petri dish with differentiation medium consisting of DMEM (Invitrogen) supplemented with 10% of FBS (Hyclone). The doubling time of oiPSCs was determined as described by Kim 41. The plating efficiency was determined as described by Amit et al. 42.
Lentiviral transduction and reprogramming culture
Human cDNAs were inserted downstream of the tet operator in a lenti-vector. On day 0, 0.1 million sheep fibroblast cells were transduced with a lentivirus carrying GFP (negative control) or a cocktail of lentiviruses carrying reprogramming factors. At 1 day after transduction, the cells were harvested by trypsinization and plated onto MEFs at 2.5 × 104 cells per well in a six-well plate. The next day, the medium (DMEM containing 10% FBS) was replaced with ES medium plus DOX (1 μg/ml). On days 11 and 17, the cells were trypsinized into a single-cell suspension and passaged twice. ESC-like colonies were picked on day 23 and plated onto new culture dishes with feeder cells.
Immunostaining
Immunostaining was carried out similarly to previously described methods 43. The primary antibodies used were anti-Oct4 (1:100, Santa Cruz), anti-Sox2 (1:1 000, Millipore), anti-Nanog (1:150, Santa Cruz), anti-SSEA1 (Ascites, 1:500, Developmental Studies Hybridoma Bank), anti-SSEA3 (Ascites, 1:400, Developmental Studies Hybridoma Bank), anti-SSEA4 (Ascites, 1:400, Developmental Studies Hybridoma Bank), anti-Tra-1-60 (1:150, Chemicon), anti-Tra-1-81 (1:150, Chemicon), anti-Rex1 (1:200, Santa Cruz), anti-E-cadherin (1:100, BD), anti-βIII-tubulin (1:100, Santa Cruz), anti-SMA (1:200, Santa Cruz) and anti-Sox17 (1:100, R&D).
Real-time PCR
Total RNA was prepared using an RNeasy kit (Qiagen) and reverse transcribed to synthesize first-strand DNA. The cDNA was used as a template for RT-PCR. Real-time PCR was performed in an Eppendorf Mastercycler real-time PCR system using a SYBR Green-based PCR Master mix (TOBOYO). The PCR primers are listed in Supplementary information, Table S1. The CT data for the gene of interest of each sample that were obtained from the real-time PCR were normalized to the internal control (GAPDH), and the fold changes of iPS samples relative to fibroblasts were calculated.
Bisulphite genomic sequencing
Bisulphite treatment was performed using a CpGenome modification kit (Chemicon) according to the manufacturer's recommendations. The PCR primers are listed in Supplementary information, Table S2. The amplified products were cloned into the T-vector, and at least 10 randomly selected clones were sequenced.
Karyotype analysis
Karyotyping was performed at the Xiangtan Center Hospital using standard protocols for high-resolution G-banding.
Teratoma formation
The oiPSCs were injected intramuscularly into NOD/SCID mice (approximately 5 × 106 cells per site). After 4-6 weeks, tumors were processed for hematoxylin and eosin staining. All animal experiments were conducted in accordance with the Guide of SIBS for the Care and Use of Animals for Research Purposes and were approved by the SIBS Animal Care Committee.
References
Campbell KH, McWhir J, Ritchie WA, Wilmut I . Sheep cloned by nuclear transfer from a cultured cell line. Nature 1996; 380:64–66.
Damak S, Jay NP, Barrell GK, Bullock DW . Targeting gene expression to the wool follicle in transgenic sheep. Biotechnology (NY) 1996; 14:181–184.
Damak S, Su H, Jay NP, Bullock DW . Improved wool production in transgenic sheep expressing insulin-like growth factor 1. Biotechnology (NY) 1996; 14:185–188.
Wall RJ, Rexroad CE, Jr, Powell A, et al. Synthesis and secretion of the mouse whey acidic protein in transgenic sheep. Transgenic Res 1996; 5:67–72.
Evans MJ, Kaufman MH . Establishment in culture of pluripotential cells from mouse embryos. Nature 1981; 292:154–156.
Thomas KR, Capecchi MR . Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 1987; 51:503–512.
Capecchi MR . Altering the genome by homologous recombination. Science 1989; 244:1288–1292.
Martin GR . Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 1981; 78:7634–7638.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145–1147.
Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA 1995; 92:7844–7848.
Buehr M, Meek S, Blair K, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell 2008; 135:1287–1298.
Li P, Tong C, Mehrian-Shai R, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell 2008; 135:1299–1310.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131:861–872.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663–676.
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318:1917–1920.
Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007; 318:1920–1923.
Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007; 448:318–324.
Liao J, Cui C, Chen S, et al. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell 2009; 4:11–15.
Li W, Wei W, Zhu S, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 2009; 4:16–19.
Wu Z, Chen J, Ren J, et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol 2009; 1:46–54.
Esteban MA, Xu J, Yang J, et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem 2009; 284:17634–17640.
Ezashi T, Telugu BP, Alexenko AP, et al. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci USA 2009; 106:10993–10998.
Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008; 451:141–146.
Mali P, Ye Z, Hommond HH, et al. Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells 2008; 26:1998–2005.
Chou YF, Chen HH, Eijpe M, et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell 2008; 135:449–461.
Chen T, Yuan D, Wei B, et al. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells 2010; 28:1315–1325.
Li R, Liang J, Ni S, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell; 7:51–63.
Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 2008; 321:699–702.
Zhao XY, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009; 461:86–90.
Kang L, Wang J, Zhang Y, Kou Z, Gao S . iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 2009; 5:135–138.
Ahuja D, Saenz-Robles MT, Pipas JM . SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 2005; 24:7729–7745.
Cheng L, Kelly TJ . Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell 1989; 59:541–551.
Erdile LF, Collins KL, Russo A, et al. Initiation of SV40 DNA replication: mechanism and control. Cold Spring Harb Symp Quant Biol 1991; 56:303–313.
Bocchetta M, Eliasz S, De Marco MA, et al. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res 2008; 68:1022–1029.
Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 2009; 460:1132–1135.
Kawamura T, Suzuki J, Wang YV, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 2009; 460:1140–1144.
Marion RM, Strati K, Li H, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 2009; 460:1149–1153.
Utikal J, Polo JM, Stadtfeld M, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 2009; 460:1145–1148.
Zhao Y, Yin X, Qin H, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell 2008; 3:475–479.
Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279:349–352.
Kim DK . Estimating doubling time of cells in vitro. In Vitro Cell Dev Biol Anim 1995; 31:419–420.
Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 2000; 227:271–278.
Xiao L, Yuan X, Sharkis SJ . Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells 2006; 24:1476–1486.
Acknowledgements
This research was partially supported by grants from the National Key Basic Research and Development Program of China (2007CB947902, 2007CB948003, 2009CB941101, 2009CB940903), the National Natural Science Foundation of China (30971682, 30901019, 31025016), the Ministry of Agriculture (2009ZX08010-016B), the National High Technology Research and Development Program (863 program) of China (2010AA100504) and the Chinese Academy of Sciences Knowledge Creation Program (KSCX2-YW-R-223).
Author information
Authors and Affiliations
Corresponding author
Additional information
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Immunostaining of OCT4, SOX2 and NANOG in sheep iPS cells. (PDF 57 kb)
Supplementary information, Table S1
Primers for RT-PCR (PDF 17 kb)
Supplementary information, Table S2
PCR primers for Bisulfite genomic sequencing (PDF 28 kb)
Rights and permissions
About this article
Cite this article
Bao, L., He, L., Chen, J. et al. Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors. Cell Res 21, 600–608 (2011). https://doi.org/10.1038/cr.2011.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2011.6
Keywords
This article is cited by
-
Establishment of Capra hircus somatic cells and induction of pluripotent stem-like cells
In Vitro Cellular & Developmental Biology - Animal (2024)
-
The impact of induced pluripotent stem cells in animal conservation
Veterinary Research Communications (2024)
-
The miR-302s/367 Cluster Inhibits the Proliferation and Apoptosis in Sheep Fetal Fibroblasts via the Cell Cycle and PI3K-Akt Pathways
Mammalian Genome (2021)
-
The use of induced pluripotent stem cells in domestic animals: a narrative review
BMC Veterinary Research (2020)
-
Generation of induced pluripotent stem cells from large domestic animals
Stem Cell Research & Therapy (2020)