Abstract
1-Deoxy-D-xylulose-5-phosphate reductoisomerase (DXR) is an important enzyme involved in the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway which provides the basic five-carbon units for isoprenoid biosynthesis. To investigate the role of the MEP pathway in plant development and metabolism, we carried out detailed analyses on a dxr mutant (GK_215C01) and two DXR transgenic co-suppression lines, OX-DXR-L2 and OX-DXR-L7. We found that the dxr mutant was albino and dwarf. It never bolted, had significantly reduced number of trichomes and most of the stomata could not close normally in the leaves. The two co-suppression lines produced more yellow inflorescences and albino sepals with no trichomes. The transcription levels of genes involved in trichome initiation were found to be strongly affected, including GLABRA1, TRANSPARENT TESTA GLABROUS 1, TRIPTYCHON and SPINDLY, expression of which is regulated by gibberellic acids (GAs). Exogenous application of GA3 could partially rescue the dwarf phenotype and the trichome initiation of dxr, whereas exogenous application of abscisic acid (ABA) could rescue the stomata closure defect, suggesting that lower levels of both GA and ABA contribute to the phenotype in the dxr mutants. We further found that genes involved in the biosynthetic pathways of GA and ABA were coordinately regulated. These results indicate that disruption of the plastidial MEP pathway leads to biosynthetic deficiency of photosynthetic pigments, GAs and ABA, and thus the developmental abnormalities, and that the flux from the cytoplasmic mevalonate pathway is not sufficient to rescue the deficiency caused by the blockage of the plastidial MEP pathway. These results reveal a critical role for the MEP biosynthetic pathway in controlling the biosynthesis of isoprenoids.
Similar content being viewed by others
Introduction
Isoprenoids are the most functionally and structurally varied metabolites in plants, with tens of thousands of compounds reported to date 1, 2. They play a critical role in plant respiration, photosynthesis, regulation of growth and development. They also act to protect plants against herbivores and pathogens, to attract pollinators and seed-dispersing animals, and to influence competition among plant species 2. All natural isoprenoids are derived from two basic five-carbon molecules, isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP) 1, 3. In higher plants, the isoprenoid building units are formed from two pathways that operate in different subcellular compartments 4, 5, 6. One is the mevalonate (MVA) pathway, which is localized in the cytosol 7. The other is the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway, which is localized in plastids 7. Sesquiterpenes, triterpenes, cytokinins and brassinosteroids are synthesized via the MVA pathway, while carotenoids, lutein, chlorophyll side chains, ubiquinone, gibberellic acids (GAs) and abscisic acid (ABA) are synthesized via the MEP pathway (Figure 1, 7, 8, 9).
MEP pathway with plastids localization in plants (modified from Qin et al. 30). The symbol for anabolic reactions is a solid arrow. Open arrows and double solid arrows depict multiple enzymatic steps. Certain key enzymes are written in bold. G3P: glycerol triphosphate; DXP: 1-deoxy-D-xylulose-5-phosphate; MEP: 2-C-methyl-D-erythritol-4-phosphate; IPP: isopentenyl diphosphate; DMAPP: dimethylallyl diphosphate; GPP: geranyl diphosphate; GGPP: geranyl geranyl diphosphate; DXS: 1-deoxy-D-xylulose-5-phosphate synthase; DXR: 1-deoxy-D-xylulose-5-phosphate reductoisomerase; IPI: isopentenyl diphosphate; GGPS: geranylgeranyl diphosphate synthase; GGRS: geranylgeranyl reductase; PSY: phytoene synthase; PDS: phytoene desaturase; ZDS: ξ-carotene desaturase; LYC: lycopene cyclase; CPS: copalyl diphosphate synthase; KO: ent-kaurene oxidase.
The MVA pathway has been extensively studied since its discovery in the 1950s, and has been reviewed in detail 1, 3. Different from the MVA pathway, the MEP pathway was first identified in bacteria about 15 years ago and later in plants 6, 10. Currently, all the enzymes involved in the MEP pathway have been identified. In the first step of this pathway, 1-deoxy-D-xylulose-5-phosphate synthase (DXS) catalyzes the formation of 1-deoxy-D-xylulose-5-phosphate (DXP) from pyruvate and D-glyceraldehyde-3-phosphate 11, 12, 13. Then, DXP is transformed to MEP, catalyzed by DXR 14. Finally, MEP is converted to IPP and DMAPP after consecutive steps catalyzed by 2-C-methyl-D-erythritol-4-phosphate cytidylyltransferase (CMS), 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase (CMK), 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase (MCS), 4-hydroxy-3-methylbut-2-enyl diphosphate synthase (HDS), 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR) and isopentenyl diphosphate isomerase (IPI) 9, 5, 15, 16.
In the MEP pathway, MEP serves as the precursor not only for IPP or DMAPP, but also for vitamin B1 17, 18. Previous studies have shown that DXR played a critical role in directing the intermediate flux into IPP and DMAPP biosynthetic pathways 19, 20. Over-expression of DXR resulted in an increase in biosynthesis of essential oils in mint and an increase in photosynthetic pigment levels in Arabidopsis 20, 21. All results suggest that DXR contributes more specifically to the MEP pathway than DXS. The Arabidopsis genome contains only one DXR gene. The transcript pattern of DXR covers almost all plant organs, with the highest level in inflorescence tissue 19, 22. Blocking of DXR activity using the specific inhibitor fosmidomycin (FSM) led to the accumulation of DXR protein, the bleach phenotype, and the failure of seedling establishment 19, 20, 23, 24, 25, 26. An insertion mutant of DXR was found to be an albino, and down-regulation of DXR transcript level resulted in abnormal chloroplast development 20, 27. However, until now, the phenotypic analysis was only restricted to chloroplast development and photosynthetic pigment biosynthesis.
In addition to the photosynthetic pigments, both GAs and ABA are important chemical molecules synthesized from the plastidial geranyl geranyl diphosphate (GGPP) precursor, which is derived from IPP and DMAPP of the MEP pathway 8, 9, 28, 29. The biosynthetic pathways for GA and ABA have been well documented 7. However, how the MEP pathway contributes to the biosynthesis of GA and ABA remains ambiguous, due to the complexity of IPP and DMAPP biosynthesis 7, 25. Potential crosstalk of IPP and DMAPP between the MVA and MEP pathways might affect the downstream biosynthesis, and channeling of the IPP and DMAPP precursors to different isoprenoid biosynthetic pathway branches is tightly controlled. Previous studies indicated that the amount of ent-kaurene, the important precursor for GA biosynthesis, was decreased in plants with reduced DXR activity 25. Exogenous application of GAs to the medium could partially rescue the dwarf phenotype of the pds3 mutant 30. Other research indicated that levels of not only GAs but also other important compounds were decreased as a result of the metabolic disruption in the mutants of enzymes involved in the MEP pathway 21, 30, 31, 32, 33, 34. However, no detailed analyses have been reported about the developmental and the physiological changes due to the complete blockage of the MEP pathway.
In order to investigate the contribution of the MEP pathway to the developmental and physiological processes in Arabidopsis, we characterized a dxr mutant and two DXR transgenic co-suppression lines in this study. The transcript changes of selected enzymes involved in the isoprenoid biosynthetic pathway were analyzed to investigate the flux regulation mechanism of plastid isoprenoid biosynthesis.
Results
Phenotypic analysis to the DXR null mutant
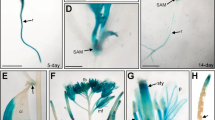
In order to understand the effects of the MEP pathway on plant development, we obtained a T-DNA insertion mutant line, GK-215C01 35. The T-DNA insertion site of GK-215C01 is localized in the seventh exon of the DXR gene (Figure 2A). RT-PCR analysis showed that the expression of DXR was abolished (Figure 2B), indicating that GK-215C01 is a null mutant, and it was thus renamed as dxr. Phenotypic analysis of dxr shows that dxr has deficiencies in multiple developmental and physiological processes. The homozygous dxr mutants display an albino phenotype and grow purple cotyledons (Figure 2C). They grow round and small true leaves with very short petioles, and never bolt during their whole life-time (Figure 2C). The growth of dxr mutants is severely retarded, since the 60-day-old dxr mutant plants have only 10 leaves, whereas the 40-day-old wild-type plants have as many as 12 leaves and begin to bolt (Figure 2D). Because the homozygous dxr mutants could not produce offspring, we could only obtain the homozygous dxr mutants from the normally growing dxr heterozygous mutant. When the homozygous mutant was transferred to the soil, it lost water almost immediately and wilted to death (data not shown). For further observations, the dxr plants had to be grown on MS plates with 1% sucrose (Figure 2C).
Identification of DXR null mutant. (A) DXR gene structure and the T-DNA insertion site in GK-215C01 mutant. (B) RT-PCR analysis for the transcription level of DXR gene in wild-type (WT) plants and GK-215C01 mutant plants. (C) dxr seedling with purple cotyledons; adult dxr mutant plants and WT plants grown on MS medium for 2 months. (D) 40 days of WT plants with 12 leaves and 60 days of homozygous dxr mutant with 10 leaves.
To investigate the fine cellular structure changes of the dxr mutant, we performed scanning electron microscope (SEM) and transmission electron microscope (TEM) analyses. The TEM results showed that, while the wild-type (WT) plants developed normal chloroplasts (Figure 3A), the dxr mutant only developed proplastids, with no normal thylakoids observed (Figure 3B), suggesting that the plastid development was blocked at the pro-plastid stage. The SEM results showed that, compared to the WT (Figure 3C, 3F and 3H), not only did dxr mutants grow reduced numbers of trichomes (Figure 3D, 3E and 3I) but also their trichomes were smaller than those of WT plants, although the trichome shapes were similar (Figure 3G). An interesting phenomenon of the dxr mutant is that the latest grown leaves are usually glabrous (Figure 3I). Besides the trichome developmental phenotype, we also found that, compared with the WT (Figure 3J), the stomata of the dxr mutant had a larger opening (Figure 3K), indicating that the dxr stomata closed abnormally.
Detailed phenotypes of wild-type (WT) plants (A, C, F, H, J) and dxr null mutants (B, D, G, I, K). (A, B) The development of plastids in WT and dxr mutant plants, bar = 2.2 μm. (C-E) The statistical analyses of trichome number on four leaves of WT and dxr mutant, WT: 53 ± 3.7 (n ≥ 10), dxr: 6.2 ± 2.4 (n ≥ 10); in (C, D), bar = 1 cm. (C, F, H) The trichome development of WT Arabidopsis plant. (D, G, I) The trichome development of dxr null mutant. In (F, G), bar = 200 μm; in (H, I), bar = 300 μm. (J, K) The stomata of WT and dxr mutant plants, bar = 100 μm.
Over-expression of DXR in Arabidopsis led to transgene-induced gene silencing
We constructed an over-expression vector for the DXR gene (Figure 4A). Attempts to create DXR over-expression lines in Arabidopsis resulted in some transformants growing pale green or albino inflorescences (Figure 4B and 4C, and Supplementary information, Figure S1). These tissue-specific pale green or albino phenotypes were stably and regularly inherited in two lines, OX-DXR-L2 and OX-DXR-L7. In order to investigate whether this stable phenotype was related to transgene-induced gene silencing, the DXR transcript level was analyzed in green leaves and pale green or albino inflorescences from either OX-DXR-L2 or OX-DXR-L7, respectively. The results showed that DXR expression is up-regulated in green leaves, but down-regulated in the gray or albino inflorescences of the same plant (Figure 4D). A much more severe decrease of the DXR transcript level was detected in the albino inflorescences of OX-DXR-L7 plants than in those of OX-DXR-L2 plants (Figure 4D). Accordingly, the contents of photosynthetic pigments were also measured in different tissues of both OX-DXR-L2 and OX-DXR-L7 lines. In OX-DXR-L2 inflorescences, chlorophyll a (Chl a) was reduced to 93.2% of the WT level. The levels of chlorophyll b (Chl b) and carotenoids decreased to 47.3% and 48.5% of the WT levels, respectively (Figure 4F). In OX-DXR-L7 inflorescences, the levels of Chl a, Chl b and carotenoids were reduced to 72%, 29.5% and 42.3% of the corresponding WT levels, respectively (Figure 4F). However, in leaves with increased levels of DXR the contents of photosynthetic pigments did not change much (Figure 4E), suggesting that the metabolic balance was more sensitive to a reduction in the DXR transcript level than an increase.
Photosynthetic pigments level in DXR co-suppressed inflorescence. (A) Structure of the construct to over-express DXR in Arabidopsis plants. (B) Green inflorescence and flower buds from WT plants. (C) Albino inflorescence and flower buds from OX-DXR-L7 plants. (D) Co-suppressed DXR transcript level in OX-DXR-L2 and OX-DXR-L7 plants, in comparison with WT plants. (E) Photosynthetic pigments level of green leaves from WT, OX-DXR-L2, and OX-DXR-L7 plants. (F) Photosynthetic pigments level of inflorescence from WT, OX-DXR-L2, and OX-DXR-L7 plants. In (E, F), values represent means and standard deviations from populations of at least 20 individuals in three independent biological repeats.
As the dxr mutant did not develop flowers, the DXR co-suppression inflorescences provided an excellent model to analyze the effect of the MEP pathway on the flower development process. Consistent with the observation from the dxr leaves, OX-DXR-L7 also showed a phenotype of reduced trichome number on the sepal of the albino flower (Figure 4C). The albino flower buds were smaller and displayed difficulty in opening (Supplementary information, Figure S1).
DXR transcript level affects the transcription of many genes involved in carotenoid, chlorophyll, GA and ABA biosynthesis pathways
In many cases, the changed expression levels of enzymes induce complicated metabolic changes, which consequently result in multiple developmental or physiological deficiencies in plants. Previous research has shown that mutants of enzymes involved in the MEP pathway always show feedback regulation 30, 32. It was thus reasonable to hypothesize that the same kind of metabolic changes might exist in the dxr mutant or tissues with reduced DXR expression levels.
To investigate this possibility, 10 genes, i.e., DXS, GGPS, GGRS, IPI, GA3, PDS1, PDS3, ZDS, LYC and PSY, were chosen 7, and their transcript levels were examined. DXS catalyzes the first committed step of the MEP pathway (Figure 1). DXS and DXR are both shown to be related to flux regulation in the MEP pathway. GGRS, GGPS and IPI are enzymes acting at the steps close to the branching points of the MEP pathway (Figure 1). GA3 is shown to be involved in GA3 biosynthesis. PDS, ZDS and LYC regulate the biosynthesis of all kinds of carotenoids (Figure 1). Some of those carotenoids can be converted into ABA (Figure 1). In DXR over-expressed leaf tissue, many of the genes examined showed an increase in transcript level. The PDS3 gene had the highest increase to about 2.2 times that of the WT level (Figure 5). However, in gene-silenced albino flower tissues, the transcript level of all these genes decreased. The transcript level of GGPS was most significantly reduced, to about 19.1% of the WT level (Figure 5). Notably, there was an obvious decrease in GA3 transcript level, suggesting that the biosynthesis of GA was reduced (Figure 5). ABA biosynthesis might also be reduced due to the decrease in the transcript levels of genes involved in carotenoid biosynthesis (Figure 5). The above data indicate that the MEP pathway and downstream metabolic pathways are coordinately regulated through the regulation of the expression levels of key enzymes involved.
Trichome defects in dxr might result from abnormal GA-regulated initiation and endo-reduplication
Trichomes develop in four steps: initiation, endo-reduplication, branching and expansion 36, 37, 38, 39, 40, 41, 42. To assess the developmental deficiencies of trichome in dxr, we examined the expression of the regulators involved in different steps of trichome development. These regulators include GLABRA1 (GL1), GL3, TRIPTYCHON (TRY), TRANSPARENT TESTA GLABROUS 1 (TTG1), SPINDLY (SPY), TUBULIN FOLDING FACTOR A (TFCA), TUBULIN FOLDING FACTOR C (TFCC), KAKTUS (KAK), WASP family Verprolin-homologous protein 1 (WAVE1), WAVE3, and WAVE4. GL1, GL3, GL2, and TTG1 are known as positive regulators of trichome initiation, while TRY acts as an important negative regulator in this step. Both SPY and KAK are involved in negative regulation of trichome endo-reduplication. TFCA and TFCC are positive regulators of the trichome branching step. WAVE1, WAVE3, and WAVE4 can positively regulate the final trichome expansion step.
Our quantitative real-time PCR results revealed obvious expression changes of several important genes in the dxr mutant (Figure 6). The positive regulators of trichome initiation such as GL1 and TTG1 had an obvious decrease in their transcript levels, and the expression level of the negative regulator TRY was highly increased, consistent with the observed phenotype of reduced trichome initiation. Both SPY and KAK had an increase in their expression levels, suggesting a potential defect in trichome endo-reduplication. Notably, most of these genes were strongly regulated by GA3. When 10 μM GA3 was applied, the transcript levels of GL1, TTG1, and SPY significantly increased or decreased, in a tendency opposite to that in dxr mutant (Figure 7). This result is consistent with the important role of GAs in trichome formation and development 43, 44, 45.
Expression analyses of regulators for trichome development. Values represent means and standard deviations from at least two independent experiments (only one representative one is shown here), each with three biological replicates. Asterisk marks the gene with significant change at the transcription level.
Expression analyses of regulators for trichome development in plants with or without GA3 treatment. Values represent means and standard deviations from at least two independent experiments (only one representative one is shown here), each with three biological replicates. Asterisk marks the gene with significant change at the transcription level.
Exogenous application of GA3 could partially rescue trichome initiation in dxr mutant
In order to further assess the relationship between dxr phenotypes and GAs, the dxr mutant was treated with exogenous GA3. 7-Day-old homozygous dxr seedlings with open cotyledons were transferred onto the MS medium supplied with 10 μM GA3 and grown for 3 weeks. It is evident that the plants grew higher and faster. The leaves expanded, and leaf petioles grew longer. Most plants could bolt and developed inflorescences with normal flower organs (Figure 8A and 8C). Meanwhile, the trichome initiation defect of dxr mutant could be partially rescued (Figure 8B). This result suggests that the phenotypes in the dxr mutant may partially result from GA deficiency, and that other metabolites synthesized from the MEP pathway might also be involved in regulating trichome development.
Exogenous application of GA3 on dxr mutant. (A) dxr mutant without GA3 treatment and dxr mutant with 10 μM GA3 treatment. The flower developed from dxr mutant treated with 10 μM GA3 was also shown. Bar = 80 μM. (B) Statistical analyses of trichome number on WT and dxr leaves with or without GA3 treatment. WT: 53 ± 3.7 (n ≥ 10), dxr: 5.75 ± 2.5 (n ≥ 10), dxr (with GA3 treatment): 22.8 ± 4.3 (n ≥ 8). (C) Measurement of petiole length of dxr mutant leaves with or without GA3 treatment. dxr: 5 mm ± 1.4 (n ≥ 10); dxr (with GA3 treatment): 11.8 mm ± 1.7 (n ≥ 10). Asterisks mark significant changes.
Exogenous application of ABA could completely rescue the stomata closure phenotype in dxr mutant
ABA, the other important phytohormone synthesized by the MEP pathway, plays a major role in the regulation of stomata opening and closing. In order to find out whether the stomata-closing defects were caused by a deficiency in ABA, 20-day-old dxr seedlings were transferred to the MS medium supplied with 5 μM ABA, and grown for 5 days before they were analyzed for the stomata structure by SEM. The result showed that in the presence of exogenous ABA the stomata closed normally in dxr mutants (Figure 9A). This was also revealed by statistical analysis of the corresponding changes of stomata structure (Figure 9B). This result suggests that ABA deficiency is the cause for the stomata-closing defects in the dxr mutant.
Exogenous application of ABA on dxr mutant. (A) Stomata structure of WT plants and dxr mutant with or without ABA treatment. (B) Statistical analyses to the stomata structure (width/length) of WT plants and dxr mutant with or without ABA treatment. WT: 0.48 ± 0.05; dxr: 0.83 ± 0.2; dxr (with ABA treatment): 0.14 ± 0.04. More than 30 stomatas from at least seven different leaves were measured for each sample. Asterisks mark significant changes.
Discussion
In plants, the MEP pathway provides the five-carbon units, IPP and DMAPP, for the biosynthesis of many important isoprenoids 29. It has been shown that disturbance to the down-stream steps of MEP-related isoprenoid biosynthetic pathways seriously affects plant development 46.
In this study, with an attempt to completely block the whole MEP pathway, we found that the dxr mutant plants displayed severe developmental defects, for instance, abnormal chlorophyll biosynthesis. Like the other albino mutants of the enzymes involved in MEP-related biosynthetic pathways, the dxr mutants exhibited an arrest in plastid development at early stages 20, 22, 30, 46, 47, 48. This is consistent with the increased chlorophyll a/b ratio observed in the pale inflorescence tissues, which are more sensitive to light due to the undeveloped plastids 49. In addition to the albino phenotype, we also found trichome initiation and stomata closure defects as a result of DXR disruption. It was previously reported that GAs play important roles in trichome development by regulating trichome-associated genes including GL1, GL3, and SPY 41, 42, 43, 44, 45. As GAs are a class of important products derived from MEP-related isoprenoid biosynthetic pathways, it is reasonable to link DXR disruption to the observed trichome developmental defects. However, the regulation of trichome development is complex. The interaction between plant and environmental cues is another important factor that may affect this process 44, 50. On one hand, the albino phenotype might lead to a hypersensitive response of the dxr mutants to environmental stresses. This may help explain why exogenous application of GA3 can only partially rescue the trichome initiation defect of dxr, and why the expression of trichome development regulators changes in the dxr mutant. On the other hand, the stomata closure defect in the dxr mutants suggests that the biosynthesis of ABA, an important interaction signal molecule between environment and plant 51, is abnormal. It is reasonable to predict that the environmental regulation of trichome development is possibly abnormal in the dxr mutant. ABA is also believed to be involved in plant development as an antagonist of GAs. Since gibberellins were shown to be able to interact with jasmonic acid and salicylic acid pathways to regulate trichome development 52, it would be interesting to investigate in the future whether the antagonistic interaction between GAs and ABA also plays a role in trichome development.
The regulation of flux in biosynthetic pathways is an attractive but complicated biological question. To gain more insight into the metabolic flux of the MEP pathway, we monitored the transcript changes in the dxr mutants, of those important enzymes involved in the MEP pathway and related isoprenoid biosynthetic pathways. In the albino tissues with reduced DXR transcript level, all the examined genes exhibited reduced transcription, which is similar to the gene expression pattern in the cla1/dxs and pds3 mutants 7, 29, 30. This is an interesting phenomenon, since these three enzymes catalyze different steps in the isoprenoid biosynthetic pathways, i.e., DXR and DXS catalyzing the initial two steps of the MEP pathway, and PDS3 catalyzing a down-stream reaction leading to ABA biosynthesis (Figure 1). In pds3 mutant, it was suggested that there might be a feedback control of the transcription of enzymes involved in the isoprenoid biosynthetic pathway by the accumulated phytoene, the substrate for the PDS3 enzyme 30. This feedback control indirectly led to decreased GA biosynthesis, a branch different from the ABA metabolic pathway. From this study, it seems that the expression of down-stream genes in the isoprenoid biosynthetic pathway is also feedback regulated by the insufficiency of initial substrates IPP and DMAPP (Figure 1). In the meantime, the result suggests that transcription of those enzymes involved in plastid isoprenoid biosynthesis is somehow coordinately regulated, leading to the decrease of both ABA and GA biosynthesis in the dxr mutant. A more detailed analysis on the mutants of other enzymes involved in the same pathway is needed in the future. As for the DXR over-expressing leaves, the increase in DXR transcript level did not lead to the accumulation of photosynthetic pigments. There are at least two possibilities. One is post-transcriptional regulation of DXR, such as translational modulation 19, 53. In Arabidopsis, HMGR, a critical enzyme involved in the MVA pathway, has even more complicated regulation mechanisms 26, 54, 55, 56, 57. The other possibility is that DXR might not be a determinant enzyme in the MEP pathway. In this pathway, DXS has been identified to catalyze the first step, the key step of the MEP pathway, in Arabidopsis 22, 47, 58. DXS was also found to share a similar expression pattern with DXR, and both DXS and DXR null mutants displayed an albino phenotype 7, 19, 22, 47. Many studies on comparisons between DXR and DXS have also been carried out 7, 19. Possibly, for those non-determinant enzymes such as DXR, their expression levels are normally more than needed, and metabolic changes will only be triggered after the changes of the enzymes reach a certain threshold.
Crosstalk between the MEP and MVA pathways is an interesting topic. It is generally believed that the chemical flux is mainly from MEP to the MVA pathway 7, 29, 59. In our study, without the functional MEP pathway, plants can still survive for a long period until the bolting stage. There are two possibilities. One possibility is that the chemical storage in homozygous mutant seeds is sufficient to support plant growth and development. The other possibility is that chemical flux from the MVA pathway is able to fulfil part of the biosynthesis requirement of the MEP pathway. As reported previously, sucrose can modulate the carbon flux through the MEP pathway, possibly either as a carbon source or as an energy indicator 53, 60. Taking into consideration that, without sucrose, the dxr null mutant plants are arrested at an early developmental stage, but with sucrose the mutant can proceed with the basic differentiation program and grow for more than 40 days, in which GAs, ABA or other important carotenoids are necessarily involved 61, the second possibility seems more likely. As carbon flux through the MEP pathway is completely blocked in the dxr mutant, it would be interesting to investigate the potential important role of sucrose in promoting the carbon flow from MVA pathway to the MEP pathway, facilitating the supply of the necessary amount of GAs or ABA.
It was previously reported that the MVA pathway plays a more important role than the MEP pathway during reproductive development in Arabidopsis 62, 63. Another interesting phenomenon from our study is that the heterozygous dxr mutant plants set normal homozygous seeds, suggesting that, without the MEP pathway, the gametophytes, embryo sac or later-developed homozygous embryo can develop normally, consistent with the previous reports. In other words, the possible crosstalk and flux between the MVA pathway and the MEP pathway are sufficient to fulfil the chemical requirement in germ cells and embryo development. As the main flux is shown to be normally from the MEP pathway to the MVA pathway 7, 29, 59, we suspect that the direction of the main flux may change at different developmental stages or under certain conditions. Due to the technique limitation for chemical detection in planta, it will be a great challenge to clarify the real-time image of the crosstalk flux in plants in the future.
In summary, by characterizing the phenotypes of dxr mutant and two DXR transgenic co-suppression lines, we found that complete blockage of the MEP pathway in dxr mutant results in typical carotenoid-deficiency phenotypes, including abnormal chloroplast and trichome development, possibly due to significant precursor deficiency for GAs and ABA biosynthesis. Detailed analysis of the dxr mutant provides evidence that the plastidial MEP biosynthetic pathway plays an essential role in plant development by regulating the isoprenoid biosynthetic pathway.
Materials and Methods
Plant material and growth conditions
Arabidopsis thaliana, ecotype Columbia-0, was used. The seeds of dxr mutant were requested from GABI-Kat. Plants were grown on Murashige and Skoog plates (GIBCO/BRL, Cleveland, OH, USA), pH adjusted to 5.7 with 1 M of KOH, containing 0.8% (w/v) phytoagar and 1% sucrose, or in soil in the greenhouse under long-day conditions (16-h-light/8-h-dark cycle) at 22 °C ± 1 °C. For GAs or ABA treatment, 10 μM of GA3 or 5 μM of ABA was applied to the MS medium in the plate or conical flask.
DXR cloning, vector construction and over-expression in Arabidopsis
The PCR product of the DXR full-length coding region was cloned into pBlueScript SK(+/−) (Stratagene, La Jolla, CA, USA) and sequenced. The DXR fragment cut by BamHI and SalI restriction enzymes was then constructed into the plant expression vector, pQG110, driven by a Cauliflower Mosaic Virus (CaMV) 35S promoter. The construct was transformed into Agrobacterium tumefaciens GV3101/pMP90. Arabidopsis plants grown in soil for one and a half months were transformed using the floral dip method 64. Transgenic plants screening was carried out as previously reported 65. Their identities were later confirmed by PCR using the DXR-specific primers: 5′-ATG ATG ACA TTA AAC TCA CTA TCT CCA G-3′ and 5′-TCA TGC ATG AAC TGG CC TAG CAC CA-3′. Seeds were collected at maturity and stored at 4 °C.
RNA extraction and reverse transcription
Total RNA from the frozen material was extracted using TRIzol Reagent. To eliminate the contamination of genomic DNA, total RNA was then treated with RNase-free DNase (Takara). In all, 5 μg of total RNA was reverse-transcribed using the SuperscriptΠ RT Kit (Invitrogen, Carlsbad, CA, USA), in a reaction of 20 μl. The cDNA was diluted 50 times and later used as the template for RT-PCR 66.
Quantitative real-time RT-PCR
The PCR amplification was performed on a MJ Research thermocycler, using a DyNAmo SYBR Green qPCR kit from Finnzymes (distributed by MJ research, incorporated; F-400L). Each reaction was carried out on a 1 μl diluted cDNA sample, in a total reaction system of 10 μl. The procedure of the reaction was set according to the manufacturer's protocol. To check the specificity of amplification, the melting curve of the PCR products was determined. The expression levels of different genes were standardized to the constitutive expression level of Tubulin2.
In one real-time PCR experiment, at least three values were produced for each sample, as previously reported 67. The relative expression level of each gene, corresponding to the expression level of Tubulin2, was calculated using the 2−ΔΔt method 68. The primers used for the quantitative real-time PCR are listed in Supplementary information, Table S1.
Chlorophyll and carotenoid analysis
Chlorophyll and carotenoids were extracted from A. thaliana leaves and inflorescence using a method modified by Gitelson et al. 69. Briefly, 4th∼7th rosette leaves harvested from 3-week-old plants or the inflorescences from 6-week-old plants were put into a pre-chilled tube, and ground for 3 min in 1 ml extraction buffer (85% acetone: Tris-HCl [1%, w/v]). After the pigments were completely extracted by the buffer, an additional 1 ml extraction buffer was used to wash the pestle. All extraction solutions were combined and debris was removed by centrifugation. A volume of 1 ml of the supernatant was diluted to 3 ml final solution. The light absorbance of the final solution at 663, 647 and 470 nm was measured. The concentrations of carotenoids and chlorophyll were calculated as described 70.
Transmission electron microscope
TEM was conducted following the procedure described by Hsieh and Goodman, with a few modifications 30, 60. The seedling samples were fixed in 4% glutaraldehyde overnight, and post-fixed with 1% osmium tetroxide for 30 min. The fixed samples were dehydrated through a series of alcohol solutions and embedded in Spurr resin. The ultra-thin sections were cut on a Reichert Ultracut-S (Leica Microsystems, Bannockburn, IL, USA) and stained with uranyl acetate and lead citrate before being viewed with a TEM (Hitachi, Tokyo, Japan).
Scanning electron microscope
SEM analysis was performed as described previously 71. The samples were viewed using a SEM.
References
Chappell J . The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol 1995; 107:1–6.
Chappell J . The genetics and molecular genetics of terpene and sterol origami. Curr Opin Plant Biol 2002; 5:151–157.
McGarvey DJ, Croteau R . Terpenoid metabolism. Plant Cell 1995; 7:1015–1026.
Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A . The deoxy-xylulose phosphate pathway of the terpenoid biosynthesis in plants and microorganisms. Chem Biol 1998; 5:R221–R233.
Lichtenthaler HK . The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 1999; 50:47–65.
Rohmer M . The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 1999; 16:565–574.
Laule O, Fürholz A, Chang HS, et al. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 2003; 100:6866–6871.
Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH . Terpenoids biosynthesis from 1-deoxy-D-xylulose in higher plants by intramolecular skeletal rearrangement. Proc Natl Acad Sci USA 1997; 94:10600–10605.
Eisenreich W, Rohdich F, Bacher A . Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 2001; 6:78–84.
Rohmer M, Knani M, Simonin P, Sutter B, Sahm H . Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J 1993; 295:517–524.
Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H . Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc 1996; 118:2564–2566.
Sprenger GA, Schörken U, Wiegert T, et al. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-D-xylulose 5-phosphate precursor to isoprenoids, thiamine, and pyridoxol. Proc Natl Acad Sci USA 1997; 94:12857–12862.
Lois LM, Campos N, Putra SR, Danielsen K, Rohmer M, Boronat A . Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of D-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc Natl Acad Sci USA 1998; 95:2105–2110.
Takahashi S, Kuzuyama T, Watanabe H, Seto H . A 1-deoxy-D-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-D-erythritol 4-phosphate in an alternative non-mevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci USA 1998; 95:9879–9884.
Hecht S, Eisenreich W, Adam P, et al. Studies on the non-mevalonate pathway to terpenes: the role of the GcpE (IspG) protein. Proc Natl Acad Sci USA 2001; 98:14837–14842.
Hintz M, Reichenberg A, Altincicek B, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett 2001; 509:317–322.
Julliard JH, Douce R . Biosynthesis of the thiazole moiety of thiamin (vitamin B1) in higher plant chloroplasts. Proc Natl Acad Sci USA 1991; 88:2042–2045.
Fitzpatrick TB, Amrhein N, Kappes B, Macheroux P, Tews I, Raschle T . Two independent routs of de novo Vitamin B6 biosynthesis: not that different at all. Biochem J 2007; 407:1–13.
Carretero-Paulet L, Cunillera N, Rodrguez-Concepcio M, Ferrer A, Boronat A . Expression and molecular analysis of the Arabidopsis DXR gene encoding 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Plant Physiol 2002; 129:1581–1591.
Carretero-Paulet L, Cairó A, Botella-Pavía P, et al. Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants over-expressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol Biol 2006; 62:683–695.
Mahmoud SS, Croteau RB . Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proc Natl Acad Sci USA 2001; 98:8915–8920.
Estévez JM, Cantero A, Reindl A, Reichler S, León P . 1-deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 2001; 276:22901–22909.
Zeidler J, Schwender J, Müller C, et al. Inhibition of the non-mevalonate 1-deoxy-D-xylulose-5-phosphate pathway of plant isoprenoid biosynthesis by fosmidomycin. Z Naturforsch 1998; 53:980–986.
Rodríguez-Concepción M, Ahumada I, Diez-Juez E, et al. 1-deoxy-D-xylulose 5-phosphate reductoisomerase and plastid isoprenoid biosynthesis during tomato fruit ripening. Plant J 2001; 27:213–222.
Okada K, Kawaide H, Kuzuyama T, Seto H, Curtis I, Kamiya Y . Antisense and chemical suppression of the non-mevalonate pathway affects ent-kaurene biosynthesis in Arabidopsis. Planta 2002; 215:339–344.
Rodríguez-Concepción M, Martínez-García JF, González V, Phillips MA, Ferrer A, Boronat A . Distinct light-mediated pathways regulate the biosynthesis and exchange of isoprenoid precursors during Arabidopsis seedling development. Plant Cell 2004; 16:144–156.
Budziszewski GJ, Lewis SP, Glover LW, et al. Arabidopsis genes essential for seedling viability: isolation of insertional mutants and molecular cloning. Genetics 2001; 159:1765–1778.
Sun TP, Kamiya Y . The Arabidopsis GA1 locus encodes the cyclase ent-kaurence synthetase A of gibberellin biosynthesis. Plant Cell 1994; 6:1509–1518.
Rodríguez-Concepción M . Early steps in isoprenoids biosynthesis: multilevel regulation of the supply of common precursors in plant cells. Phytochemistry Rev 2006; 5:1–15.
Qin G, Gu H, Ma L, et al. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chlorophyll, carotenoid, and gibberellin biosynthesis. Cell Res 2007; 17:471–482.
Botella-Pavía P, Besumbes Ó, Phillips MA, Carretero-Paulet L, Boronat A, Rodríguez-Concepción M . Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 2004; 40:188–199.
Page JE, Hause G, Raschke M, et al. Functional analysis of the final steps of the 1-deoxy-D-xylulose 5-phosphate (DXP) pathway to isoprenoids in plants using virus-induced gene silencing. Plant Physiol 2004; 134:1401–1413.
Barta C, Loreto F . The relationship between the methyl-erythriol phosphate pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiol 2006; 141:1676–1683.
Dong H, Deng Y, Mu J, et al. The Arabidopsis Spontaneous Cell Death1 gene, encoding a ζ-carotene desaturase essential for carotenoid biosynthesis, is involved in chloroplast development, photoprotection and retrograde signaling. Cell Res 2007; 17:458–470.
Li Y, Rosso MG, Strizhov N, Viehoever P, Weisshaar B . GABI-Kat SimpleSearch: a flanking sequence tag (FST) database for the identification of T-DNA insertion mutants in Arabidopsis thaliana. Bioinformatics 2003; 19:1441–1442.
Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD . A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell 1991; 67:483–493.
Walker AR, Davison PA, Bolognesi-Winfield AC, et al. The TTG1 (transparent testa, glabra1) locus which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis encodes a WD40-repeat protein. Plant Cell 1999; 11:1337–1350.
Payne CT, Zhang F, Lloyd AM . GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 2000; 156:1349–1362.
Wang S, Wang JW, Yu N, et al. Control of plant trichome development by a cotton fiber MYB gene. Plant Cell 2004; 16:2323–2334.
Schellmann S, Hülskamp, M . Epidermal differentiation: trichomes in Arabidopsis as a model system. Int J Dev Biol 2005; 49:579–584.
Gan Y, Yu H, Peng J, Broun P . Genetic and molecular regulation by DELLA proteins of trichome development in Arabidopsis. Plant Physiol 2007; 145:1031–1042.
Silverstone AL, Tseng T, Swains SM, et al. Functional analysis of SPINDLY in gibberellin signaling in Arabidopsis. Plant Physiol 2007; 143:987–1000.
Perazza D, Vachon G, Herzog M . Gibberellins promote trichome formation by up-regulating GLABROUS1 in Arabidopsis. Plant Physiol 1998; 117:375–383.
Hülskamp M . Plant trichomes: a model for cell differentiation. Nat Rev Mol Cell Biol 2004; 5:471–480.
Ishida T, Kurata T, Okada K, Wada T . A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 2008; 59:365–386.
Bouvier F, Rahier A, Camara B . Biogenesis, molecular regulation and function of plant isoprenoids. Prog Lipid Res 2005; 44:357–429.
Estévez JM, Cantero A, Romero C, et al. Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D-erythritol-4-phospate pathway in Arabidopsis. Plant Physiol 2000; 124:95–103.
Hsieh MH, Chang CY, Hsu SJ, Chen JJ . Chloroplast localization of methylerythritol 4-phosphate pathway enzymes and regulation of mitochondrial genes in ispD and ispE albino mutants in Arabidopsis. Plant Mol Biol 2008; 66:663–673.
Reger BJ, Krauss RW . The photosynthetic response to a shift in the chlorophyll a to chlorophyll b ratio of Chlorella. Plant Physiol 1970; 46:568–575.
Gianfaga TJ, Carter CD, Sacalis JN . Temperature and photoperiod influence trichome density and sesquiterpene content of Lycopersicon hirsutum f. hirsutum. Plant Physiol 1992; 100:1403–1405.
Buchanan BB, Gruissem W, Jones RL . Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists, 2000.
Traw MB, Bergelson J . Interaction effects of jasmonic acid, salicylic acid and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol 2003; 133:1–9.
Cordoba E, Salmi M, León P . Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J Exp Bot 2009; 60:2933–2943.
Dale S, Arro M, Becerra B, et al. Bacterial expression of the catalytic domain of 3-hydroxy-3-methylglutaryl-CoA reductase (isoform HMGR1) from Arabidopsis thaliana, and its inactivation by phosphorylation at Ser577 by Brassica oleracea 3-hydroxy-3-methylglutaryl-CoA reductase kinase. Eur J Biochem 1995; 233:506–513.
Learned RM . Light suppresses 3-hydroxy-3-methylglutaryl-CoA reductase gene expression in Arabidopsis thaliana. Plant Physiol 1996; 110:645–655.
Newman JD, Chappell J . Isoprenoid biosynthesis in plants: carbon partitioning within the cytoplasmic pathway. In: Parish EJ, Nes WD, eds. Biochemistry and Function of Sterols. CRC Press: Boca Raton, FL, 1997:123–134.
Kobayashi K, Suzuki M, Tang J, et al. LOVASTATIN INSENSITIVE 1, a novel pentatricopeptide repeat protein, is a potential regulatory factor of isoprenoid biosynthesis in Arabidopsis. Plant Cell Physiol 2007; 48:322–331.
Mandel MA, Feldmann KA, Herrera-Estrella L, Rocha-Sosa M, León P . CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J 1996; 9:649–658.
Bick JA, Lange BM . Metabolic cross talk between cytosolic and plastidial pathways of isoprenoids biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 2003; 451:146–154.
Hsieh MH, Goodman HM . The Arabidopsis IspH homolog is involved in the plastid non-mevalonate pathway of isoprenoid biosynthesis. Plant Physiol 2005; 138:641–653.
Ogas J, Cheng JC, Sung ZR, Somerville C . Cellular differentiation regulated by Gibberellin in the Arabidopsis thaliana pickle mutant. Science 1997; 277:91–94.
Clouse SD . Arabidopsis mutants reveal multiple roles for sterols in plant development. Plant Cell 2002; 14:1995–2000.
Suzuki M, Nakagawa S, Kamide Y, et al. Complete blockage of the mevalonate pathway results in male gametophyte lethality. J Exp Bot 2009; 60:2055–2064.
Clough SJ, Bent AF . Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 1998; 16:735–743.
Qin G, Gu H, Zhao Y, et al. Indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 2005; 17:2693–2704.
Liu J, Zhang Y, Qin G, et al. Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. Plant Cell 2008; 20:1538–1554.
Guo L, Wang Z, Lin H, et al. Expression and functional analysis of rice plasma-membrane intrinsic protein gene family. Cell Res 2006; 16:277–286.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔt method. Methods 2001; 25:402–408.
Gitelson AA, Gritz Y, Merzlyak MN . Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J Plant Physiol 2003; 160:271–282.
Lichtenthaler HK . Chlorophyll florescence signature of leave the autumnal chlorophyll breakdown. J Plant Physiol 1987; 131:101–110.
Yadegari R, De Paiva GR, Laux T, et al. Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. Plant Cell 1994; 6:1713–1729.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC Grant 90717003 to L-J Qu).
Author information
Authors and Affiliations
Corresponding author
Additional information
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary information
Supplementary information, Figure S1
Inflorescences of OX-DXR transgenic plants with co-suppressed DXR expression. (PDF 84 kb)
Supplementary information, Table S1
The primers used for the quantitative real-time PCR (PDF 59 kb)
Rights and permissions
About this article
Cite this article
Xing, S., Miao, J., Li, S. et al. Disruption of the 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR) gene results in albino, dwarf and defects in trichome initiation and stomata closure in Arabidopsis. Cell Res 20, 688–700 (2010). https://doi.org/10.1038/cr.2010.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2010.54
Keywords
This article is cited by
-
Cloning and functional analysis of the DXR gene and promoter region in Osmanthus fragrans var. semperflorens
Functional & Integrative Genomics (2023)
-
Metabolome and transcriptome analyses identify the plant immunity systems that facilitate sesquiterpene and lignan biosynthesis in Syringa pinnatifolia Hemsl.
BMC Plant Biology (2022)
-
Deficiencies in the formation and regulation of anther cuticle and tryphine contribute to male sterility in cotton PGMS line
BMC Genomics (2020)
-
Influence of sodium pyrophosphate on carvacrol biosynthesis in Satureja khuzistanica Jamzad, and its effects on antioxidant, cytotoxic and antibacterial activity against periodontal bacteria
Plant Cell, Tissue and Organ Culture (PCTOC) (2020)
-
The priming fingerprint on the plant transcriptome investigated through meta-analysis of RNA-Seq data
European Journal of Plant Pathology (2020)