Abstract
MSH5, a member of the MutS homolog DNA mismatch repair protein family, has been shown to be required for proper homologous chromosome recombination in diverse organisms such as mouse, budding yeast and Caenorhabditis elegans. In this paper, we show that a mutant Arabidopsis plant carrying the putative disrupted AtMSH5 gene exhibits defects during meiotic division, producing a proportion of nonviable pollen grains and abnormal embryo sacs, and thereby leading to a decrease in fertility. AtMSH5 expression is confined to meiotic floral buds, which is consistent with a possible role during meiosis. Cytological analysis of male meiosis revealed the presence of numerous univalents from diplotene to metaphase I, which were associated with a great reduction in chiasma frequencies. The average number of residual chiasmata in the mutant is reduced to 2.54 per meiocyte, which accounts for ∼25% of the amount in the wild type. Here, quantitative cytogenetical analysis reveals that the residual chiasmata in Atmsh5 mutants are randomly distributed among meiocytes, suggesting that AtMSH5 has an essential role during interference-sensitive chiasma formation. Taken together, the evidence indicates that AtMSH5 promotes homologous recombination through facilitating chiasma formation during prophase I in Arabidopsis.
Similar content being viewed by others
Introduction
Eukaryotic cells such as those from yeast, Caenorhabditis elegans, mice, humans and Arabidopsis possess DNA mismatch repair (MMR) proteins. These MMR proteins are crucial to the fidelity of both DNA replication and the chromosome interactions that involve a series of interrelated events, including chromosome paring, synapsis and recombination. They are classified as MutS and MutL homologs, which refer to the Escherichia coli MutS and MutL post-replicative MMR model systems 1. MutS proteins are heterodimers that recognize and bind mismatched base pairs that arise from erroneous DNA replication or from recombination, whereas MutL may act as a bridging factor that binds to the DNA-MutS complex and is required for triggering excision and resynthesis of the error-containing DNA strand 2.
In the budding yeast Saccharomyces cerivisiae, there are six MutS homologs. Five of these genes (Msh2-6) encode proteins that act on nuclear DNA, whereas Msh1 is required for the stability of the mitochondrial genome 3, 4. Unlike other MutS family members, Msh4 and Msh5 do not need to recognize mismatched base pairs; instead, it has been proposed that the ability to recognize aberrant DNA structures is modulated in these proteins through evolution. They can recognize recombination intermediates such as Holliday junctions, which are four-stranded DNA structures, and reduce the free energy of these intermediates to facilitate recombination 5, 6. Mutants of Msh4 and/or Msh5 display reduced levels of meiotic crossover and spore viability by as much as 30-50% of the wild type.
Homologs of Msh4 and/or Msh5 have also been found in nematodes 7, mice 8, 9, humans 10, 11 and Arabidopsis 12. It has been reported that MSH4 and MSH5 function in mammals in a similar way to yeast; they function as heterodimers to specifically promote crossing-over during meiotic recombination. Mouse MSH5 is required for chromosome pairing and synapsis in male and female meiosis 8, 9 and can physically interact with MSH4 to form heterodimers 13. Mice that carry disrupted Msh5 have been reported to display defective chromosome synapsis, resulting in testicular and ovarian degeneration and, therefore, male and female sterility 8. In humans, genetic variations in MSH5 are associated with IgA deficiency and common variable immune deficiency, which are probably due to the regulation of Ig class switch recombination 14.
Previously, a homology-based search revealed seven MutS homologs, which were designated AtMSH1-7, and at least four MutL homologs, AtMLH1, AtMLH3, AtMLHX and AtPMS1, in Arabidopsis. AtMSH1 is involved in maintenance of the mitochondrial genome 15. AtMSH2 has an anti-recombination effect, which is a function of homolog sequence divergence 16, and is essential for maintaining nuclear genome integrity during diploid growth by limiting the accumulation of insertion/deletion mutations during seed-to-seed propagation 17. Arabidopsis plants deficient in AtMSH4 exhibited normal vegetative growth but severe reduction in fertility, which is consistent with delayed and incomplete chromosome prophase I synapsis. Moreover, metaphase I chiasma frequency was greatly reduced to as much as ∼15% of the wild type, leading to univalence and nondisjunction. However, although it is known that AtMSH2-AtMSH3, AtMSH2-AtMSH6 and AtMSH2-AtMSH7 form heterodimers and bind to various forms of DNA base pair mismatches 18, the biological function of other Arabidopsis MutS homologs, including AtMSH3, AtMSH5, AtMSH6 and AtMSH7, awaits further investigation.
In this study, we demonstrate that an Arabidopsis T-DNA insertion mutant Atmsh5 exhibits defects in male meiosis, which include the presence of numerous univalents, a random distribution of residual chiasmata and decreased levels of chiasma frequency during prophase I of meiotic division.
Results
Isolation and characterization of a T-DNA insertion Atmsh5 mutant
In our previous study of the gene expression pattern of AtNPC2 (unpublished observations), the promoter fused with the GFP-GUS gene was transformed into Arabidopsis (Columbia) via Agrobacterium-mediated transformation (Figure 1A), and tens of independent transgenic plants were obtained. One of these transgenics resembled the wild type during vegetative development under normal growth conditions, and its flowers had normal morphology, including normal floral organ identity and number (Figure 1B-1E). However, drastically reduced silique length and seed-set, compared with the wild type, was observed in this transgenic (Figure 1F-1I), which is indicative of probable fertility defects. Mean silique length was reduced to 7.5 ± 0.59 mm in the transgenic (n = 150 from 10 individual plants), compared with 12.0 ± 0.54 mm in the wild type, and the mean seed-set was only 9 ± 1.34 per silique, accounting for 16.6% of the normal seed-set in the wild-type Columbia ecotype (n = 150 from 10 individual plants). Some of the mutant seeds were abnormal in appearance with dark (red arrow) and shrunken (red arrowhead) seed coats (Figure 1I).
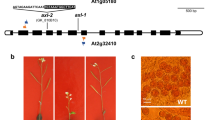
Phenotypic characterization of the T-DNA insertion mutant. (A) Schematic representation of the NPC2 promoter GFP-GUS fusion construct. The scheme illustrates the construct structure only, and is not to scale. LB and RB represent the left border and right border on T-DNA. attB1 and attB2 represent the two short stretches of sequences that participate in the recombination reaction of the Gateway system. T35S indicates 35S terminator. (B) Wild-type plant. (C) T-DNA insertion mutant plant, with vegetative growth that is similar to that of the wild type. (D) Wild-type flower. (E) T-DNA insertion mutant flower in which all flower organs appear normal. (F) Siliques of the wild-type plant. (G) Siliques of the T-DNA insertion mutant are smaller than those of the wild type. (H) A dissected silique of the wild type, showing normal developing seeds. (I) A dissected silique of the T-DNA insertion mutant, showing only a few developing seeds.
To identify the gene that is mutated in the transgenic, we sought to identify the position of the T-DNA insertion through PCR-walking (see Materials and Methods). Two PCR products were obtained using ScaI- and EcoRV- digested genomic DNA as templates. Sequencing of the two PCR products confirmed the T-DNA insertion and precisely mapped both of the products to a position 87 523 bp along the BAC clone MQC1 (locus At3g20475). Results from an NCBI blast search indicate that this locus encodes a putative Arabidopsis thaliana MutS-like protein 5 (AtMSH5), and that the T-DNA has inserted into exon 33 (Figure 2A). The T-DNA insertion was confirmed through amplification of the flanking region using the primer specific to the left border of the T-DNA and the primer specific to the genomic DNA sequences that were isolated through PCR-based walking. PCR amplification from genomic DNA of the homozygous mutant line confirmed the absence of the wild-type allele (data not shown). RT-PCR analysis using the gene-specific primers did not detect AtMSH5 transcripts even after 33 cycles of amplification in the T-DNA insertion mutant (Figure 3).
AtMSH5 gene structure and its homologs from humans, mice and C. elegans. (A) Exon/intron structure of the AtMSH5 gene. Solid black boxes represent exons. 5′ UTR and 3′ UTR are shown in solid red boxes. The triangle indicates the site of T-DNA insertion, and the two arrows show the positions of the primers that were used for RT-PCR analysis of AtMSH5 expression (Figure 3). (B) Alignments of the deduced amino-acid sequences of AtMSH5 with its homologs in humans, mice and yeast. These four MSH5 homologs were aligned using the ClustalW program from the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw/). 3-level conservation shading was accomplished using the GeneDoc program. Residues that are conserved across all four sequences are shaded black; residues that are conserved across two or three sequences are shaded light gray or dark gray, respectively. Consensus sequences are shown underneath; conserved amino-acid groups are as follows: 1 = DN, 2 = EQ, 3 = ST (hydroxylated), 4 = KR (basic), 5 = FYW (aromatic) and 6 = LIVM (aliphatic or M).
To confirm the causal relationship between disruption of the AtMSH5 gene and reduced fertility, the Atmsh5 mutant was crossed with the wild-type male or female parent, and co-segregation of the phenotype with the genotype was analyzed in the F2 progeny. As many as 92 F2 progeny were genotyped using both the T-DNA left border specific primer and the primers designed against the AtMSH5 genomic DNA sequence to detect the homozygosity of the mutated AtMSH5 gene; they were then phenotypically scored for their fertility. The results show that the mutation was monogenic and recessive, and that the T-DNA insertion in the AtMSH5 gene was tightly linked with the mutant phenotype (P < 0.001) (data not shown).
The gene structure of Arabidopsis MSH5
To identify the gene structure of the putative MSH5 in Arabidopsis, 5′- and 3′-RACE PCR analyses were performed to isolate full-length cDNA using primers specific to parts of the coding region sequences, and the results confirmed the cDNA sequences with open reading frames of 2 424 bp, released by Genbank (accession number EF471448).
Aligning the cDNA sequence against the published genomic DNA sequence of At3g20475 (TAIR) allowed the determination of the intron/exon gene structure. AtMSH5 comprises 34 exons and 33 introns (Figure 2A), of which there are 14 exons smaller than 50 bp. This is different from the predicted gene structure, which contains only 14 exons that are 990 bp long and 13 introns (NM112939). The predicted gene omits the experimentally derived exons 1-20, and the predicted 5′ UTR falls into exon 20.
AtMSH5 comprises conserved MutS domains
The AtMSH5 open reading frame encodes a predicted protein that consists of 807 amino-acid residues with a molecular mass of 91.09 kDa and a theoretical pI of 5.08. Comparison of this amino-acid sequence with other MutS homologs that are deposited in the Genbank databases indicated that it is most related to human MSH5, with 31% identity and 50% similarity (Figure 2B). It also has significant homology with MSH5 in mouse (30% identity and 50% similarity) and in yeast (29% identity and 47% similarity). A motif scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan) indicated that there is one ATP/GTP binding site (residues 569-576, also named the p-loop), and some highly conserved domains, including one MutS family signature (residues 642-658), one MutS domain II (residues 13-41), two MutS domain III (residues 180-283 and 476-520), one MutS domain IV (residues 371-392) and three MutS domain V (residues 523-610, 635-661 and 562-576). These domains are present in meiosis-specific MutS homologs from yeast, humans and C. elegans. However, AtMSH5 is devoid of MutS domain I, which is critical for MMR activity. AtMSH5 also contains an ATPase domain, encompassed in domain V, with an ATP/GTP binding site (GPNYSGKS).
AtMSH5 expression is tissue-specific
Expression of the AtMSH5 transcript was examined in different tissues using RT-PCR with gene-specific primers. Transcript expression was detected only in young floral buds at the meiotic stage, not in open flowers or vegetative tissues such as stems and leaves (Figure 3). This indicates that AtMSH5 may play an important role in meiosis during a very narrow developmental window. These results are in accordance with those from other species such as yeast, where the expression of MSH5 is detectable only during meiosis 5, and mouse, where the expression of MuMSH5 is limited to the testes and ovaries 8.
Male and female fertility is affected in Atmsh5 mutants
In order to examine the effects of Atmsh5 mutation on male and female fertility, five Atmsh5 homozygous mutant plants were used as either the female or the male parent crossed with five wild-type plants. When the mutant was the female parent, there were 8 seeds per silique on average (n = 20), among which 30% were found to be abnormal in appearance. When the mutant was the male parent, 12 seeds per silique were observed on average (n = 15), among which 33% were abnormal (dark and shrunk). These crosses indicate that both male and female fertility is impaired in the Atmsh5 mutant.
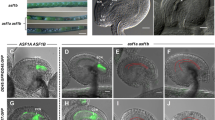
The viability of the Atmsh5 pollen grains was checked using Alexander's staining solution. Almost half of the pollen grains were not viable (376 out of 707 mutant pollen grains: 53.2%), as indicated by blue staining (Figure 4). Embryo sac development was also perturbed in the Atmsh5 mutant. In wild-type ovules, a single nuclear cell differentiates into the megaspore mother cell (MMC) (Figure 5A) and undergoes meiosis to produce four haploid megaspores, only one of which survives. This functional megaspore undergoes three mitotic divisions to produce an eight-nuclei coenocytic embryo sac, which will then cellularize into a seven-celled embryo sac (Figure 5C). Wild-type and Atmsh5 mutant pistils were investigated using confocal laser scanning microscopy (CLSM). At the MMC stage, there was no difference between the wild type and the mutant (Figure 5A and 5B). At the mature female gametophyte stage, however, only 15% (n = 500) of the mutant ovules displayed normal female gametophytes. Embryo sacs containing one functional megaspore or one cell were observed in the remaining abnormal ovules in the mutant (Figure 5D and 5E).
Pollen viabilities of the wild type and the Atmsh5 mutant. Anthers of the wild type (A) and the Atmsh5 mutant (B), which are stained with Alexander's solution. The red-purple-stained cytoplasm indicates viable pollen grains, whereas the pollen cell wall is counterstained in green and the dead pollen grains are stained blue.
Ovule development revealed by confoncal laser scanning microscopy (CLSM) in wild-type and Atmsh5 mutant plants. (A) A wild-type female gametophyte at stage FG0. (B) An Atmsh5 female gametophyte at FG0. (C) A mature ovule from a wild-type plant. (D) A one-functional megaspore embryo sac from an Atmsh5 mutant plant. (E) An ovule without embryo sac from a Atmsh5 mutant plant. MMC, megaspore mother cell; AN, antipodal nucleus; CN, central cell nucleus; EN, egg nucleus; SN, synergid nucleus; V, vacuole; FM, functional megaspore; DM, degenerating megaspore.
Disruption of AtMSH5 causes abnormal male meiosis
As the mutant had shorter siliques and less seed-set than the wild type, it is speculated that AtMSH5 may be necessary for normal meiosis. Male meiosis in wild-type and mutant plants was compared using chromosome spreads stained with 4′,6-diamidino-2-phenylindole (DAPI). In wild-type plants, male meiosis I started with the condensation of chromatin, forming distinct thin chromosome threads at the leptotene stage (Figure 6A) and undergoing synapsis at the zygotene stage (Figure 6B). Juxtaposed homologs were observed at the pachytene stage (Figure 6C). Chromosomes then underwent further condensation at the diplotene and diakinesis stages (Figure 6D and 6E) and aligned at the division plane at metaphase I (Figure 6F). At anaphase I, homologous chromosomes segregated and moved to opposite poles (Figure 6G), and eventually tetrads formed at anaphase II (Figure 6H-6L). In mutant plants, the early development stages of meiotic nuclei, in other words from leptotene to pachytene, appeared to proceed normally (Figure 7A-7C). The presence of unpaired homologous chromosomes was observed at the diplotene stage in the mutant (Figure 7D), and numerous univalents were observed at diakinesis/metaphase I (Figure 7E and 7F), which is in contrast to the five bivalents that were observed during the corresponding meiotic stages in the wild-type meiocytes (Figure 6E and 6F). At metaphase I in the mutants, the bivalents aligned at the equator of the spindle, whereas the univalents were distributed randomly (Figure 7F), behaving differently from the wild type (Figure 6F). During anaphase I, the univalents segregated abnormally, resulting in an uneven distribution of univalents (Figure 7G and 7H). At anaphase II, segregation of the sister chromotids was also disturbed (Figure 7J and 7K), characterized strikingly by the presence of abnormal numbers of chromosomes in polyads (Figure 7L). It was found that there were often three to six nuclei formed in the mutant meiotic cells in contrast to four in the wild type. Taken together, the characteristics of the resulting mutant nuclei with abnormal chromosome numbers may account for the reduction in pollen viability and fertility described previously.
Male meiosis in wild-type Arabidopsis plants. (A) Leptotene stage. (B) Zygotene stage. (C) Pachytene stage. (D) Late diplotene stage. (E) Diakinesis stage. (F) Metaphase I stage. (G) Anaphase I stage. (H) Telophase I stage. (I) Metaphase II stage. (J) Anaphase II stage. (K) Telophase II stage. (L) Tetrad stage. “a” indicates double crossover bivalents. Bar = 10 μm.
Male meiosis is disrupted in Atmsh5 mutant plants. (A) Leptotene stage. (B) Zygotene stage. (C) Pachytene stage. (D) Late diplotene stage. (E) Diakinesis stage. (F) Late metaphase I stage. (G) Anaphase I stage. (H) Telophase I stage. (I) Metaphase II stage. (J) Anaphase II stage. (K) Telophase II stage. (L) Polyads stage. “b” indicates single crossover bivalents and “u” indicates univalents. Bar = 10 μm.
The Atmsh5 mutant exhibits reduced numbers of chiasmata and bivalents
At prophase I, the mutant meiocytes exhibited a reduction in chiasma frequency compared with the wild-type meiocytes. The structure of the bivalents was used to count the chiasmata as described by Sanchez-Moran et al. 19. The distribution of the number of chiasmata per cell in the Atmsh5 mutant and in the wild type is shown graphically in Figure 8. Counts from 50 Atmsh5 mutant pollen mother cells from 5 inflorescences showed an average of 2.54 chiasmata per cell (0.51 per chromosome pair), compared with an average of 9.85 chiasmata per cell in wild-type meiocytes (1.97 per chromosome pair) (Figure 8A and 8B). In fact, 12% of Atmsh5 mutant cells lacked chiasmata completely. As expected, this resulted in frequent errors in chromosome segregation and nuclei with variable chromosome numbers from both the first and second division (Figure 7). Despite the low mean chiasma frequency in the Atmsh5 mutant, the number of chiasmata per cell was variable, ranging from 0 to 8. The number of chiasmata per wild-type meiocytes (n = 67) was close to the average, and never less than 8 nor more than 12 chiasmata per cell (Figure 8A). As numerous univalents were present in the Atmsh5 mutant meiocytes at late prophase I to metaphase I (Figure 7E and 7F), we next counted the number of bivalents on a single-celled basis. Among 50 Atmsh5 meiocytes at the diakinesis stage, 12% had no bivalents; 32%, 26%, 24%, 6% and 0% of the meiocytes were found to have 1, 2, 3, 4 and 5 bivalents, respectively, with an average of 1.8 bivalents per cell, compared with an average of 4.95 bivalents per cell in the wild type (Figure 8C).
Distribution of chiasmata and bivalents in wild-type and Atmsh5 mutant meiocytes. The frequency of chiasmata per meiocyte in the wild type (A) and in the Atmsh5 mutant (B) is shown. OD, observed distribution. PD, predicted Poisson distribution. The frequency of bivalents per meiocyte in the wild type (white bars) and in the Atmsh5 mutant (black bars) is presented in (C).
Finally and more importantly, the chiasma distribution in the Atmsh5 mutant was found to be close to the predicted Poisson distribution (χ2(6) = 9.08, P > 0.1) (Figure 8B), suggesting a near random distribution of these residual chiasmata among cells. However, in the wild type, chiasma distribution deviated significantly from the predicted Poisson distribution (χ2(11) = 117.53, P < 0.001) (Figure 8A).
Discussion
Unlike the MSH1 and MSH2 families of MutS homologs, in various organisms, including yeast, mice, C. elegans and humans, MSH5 has been reported to be essential for the interrelated and consecutive events that occur during meiosis, such as chromosome pairing, synapsis and recombination, rather than to be involved in DNA MMR in somatic cells. To our knowledge, the function of the MutS homolog MSH5 in plants has not yet been characterized. In this report, we describe the phenotype of an Arabidopsis mutant, Atmsh5. The mutant exhibits a drastic reduction in fertility, embodied by a smaller seed set and fewer viable pollen grains in the anthers than found in the wild type. Cytological observations of pollen mother cells over a range of meiotic stages revealed that abnormal meiosis occurs in the Atmsh5 mutant. In addition, quantitative cytogenetical analysis of chiasma frequency and distribution shows that the residual chiasmata in Atmsh5, about 25% of the number in the wild type, are randomly distributed among the meiocytes.
AtMSH5 contains several conserved MutS domains. MutS domain I is the mismatch recognition site, and its absence eliminates recognition of mismatched DNA bases, as evidenced by the crystal structures of MutS-DNA complexes 20, 21, 22. This domain is present in all MutS homologs and has an important role in MMR 23. The absence of MutS domain I from AtMSH5 leads us to propose that AtMSH5 may not be involved in MMR. MutS domain II is a conserved intervening region that has been proposed to have a structural role, linking MutS domain I to DNA-association domain 20, 21, 22, and resembling RNase H and RNase H-like domains 24. MutS domain III is entirely α-helical and central to the MutS structure, and is directly connected to domains II, IV and V by peptide bonds; it shares its most extensive interdomain contacts with domain V 22. MutS domain IV is involved in DNA binding by clamping DNA crossovers or Holliday junctions, and is independently folded and appended to the core of MutS by flexible peptide linkages and limited interactions, which indicates a possibility for domain rearrangement 22, 25. Domain V contains the helix-turn-helix motif that is involved in dimerization and ATPase activity 22, 26.
Loss of Msh5 function in yeast leads to a specific deficit in crossover recombination events, but cells progress through meiosis and sporulate efficiently 5. Likewise, progression through the meiotic prophase remains largely unperturbed in the C. elegans msh5 mutant, leading to completion of meiosis and consequent survival of germ cells, apart from a severe reduction in crossover frequencies and a lack of chiasmata between homologous chromosomes 7. By contrast, germ lines in the mouse msh5 mutant exhibit an arrest of meiosis at the zygotene stage, which is characterized by impaired and aberrant chromosome synapsis, and is followed by apoptotic cell death 8. These observations may indicate that there has been a functional divergence of MSH5 in various organisms.
S. cerevisiae msh4 and msh5 mutant strains show a meiotic defect that is characterized by reduced spore viability, as well as increased levels of metaphase I nondisjunction and decreased levels of reciprocal exchange between homologous chromosomes 5, 27, 28. Assessing spore viability and recombination in a yeast msh4 msh5 double mutant led to a hypothesis that, rather than working in separate pathways, Msh4 and Msh5 function in the same process 5. Therefore, Msh4 and Msh5 probably function as a heterodimeric protein complex in yeast meiotic cells 29.
Higgins et al. reported that the Arabidopsis msh4 mutant exhibits a severe reduction in fertility, which is consistent with a meiotic defect. This defect is characterized by greatly reduced chiasma frequency, leading to univalence and nondisjunction, and by delayed and incomplete prophase I chromosome synapsis 12. A similar phenotype has been described in Arabidopsis mlh3 30. However, a detailed comparison of chiasma frequencies between Atmsh4 and Atmlh3 suggests that these two classes of MMR proteins have differing roles in crossover formation, even though it has been proposed that MutL homologs combine with the MutS homologs MSH4 and MSH5 in some structural role to promote meiotic crossovers.
It has been proposed that there are two distinct classes of crossovers in S. cerevisiae 27, 31, and that these two classes are promoted by biochemically distinct pathways 32. Class I crossovers are interference sensitive and promoted by an Msh4/5-based complex, whereas class II crossovers are randomly distributed and promoted by an MMS4/MUS81-based complex. The decrease in crossover in yeast mms4 and mus81 mutants is modest, indicating that the majority of crossovers in wild-type yeast belong to class I 12, 32. Several studies have suggested the existence of two pathways in Arabidopsis. Copenhaver et al. showed that genetically determined crossover distribution better fitted the hypothesis of two pathways 33. Thereafter, two genes, AtMSH4 and AtMER3/AtRCK, were identified as the components in class I crossovers 12, 34, 35. Recently, AtMUS81 was also reported to be involved in interference-insensitive crossovers 36. Our analysis of Atmsh5 meiocytes indicates that although the number of chiasmata is greatly reduced, there is still a subset of chiasmata remaining that are independent of AtMSH5. The residual chiasmata are close to the Poisson distribution of random events, suggesting that AtMSH5 functions in the interference-sensitive pathway of meiotic recombination. The main phenotype of our mutant is similar to the Atmsh4 mutant. Therefore, evidence from our phenotype strongly supports the idea that MSH4 and MSH5 are in the same pathway and probably form a heterodimer to facilitate chiasma formation.
Material and Methods
Plant materials
The A. thaliana ecotype Columbia was used in this study for wild-type analysis. Plants were grown in a greenhouse under supplementary light (cycles of 16 h of light and 8 h of darkness) at 22 °C.
Isolation of T-DNA flanking sequence tag
The T-DNA flanking sequence was amplified as described previously 37. Two oligomers, ADPR1 and ADPR2, were mixed and incubated at 95 °C for 5 min and then at 28 °C for 5 min to allow them to anneal to form an adaptor. This adaptor was stored at −20 °C until it was used. The genomic DNA isolated from the Arabidopsis mutant was cut with blunt-end restriction enzymes, Sca I, Hpa I or Dra I, and ligated to the adaptor in the same reaction at a volume of 10 μL, with 100 ng DNA, 1 U digestion enzyme, 0.5 U T4 ligase, 1 × T4 ligase buffer and 375 nM adaptor. PCR analysis was performed using the LB1 primer together with the AP1 primer, and then nested PCR was performed using the LB2 primer together with the AP2 primer. The PCR products were directly sequenced using the LB2 primer. The oligomers used to make the adaptor are listed as follows – ADPR1: 5′-GTA ATA CGA CTC ACT ATA GGG CAC GCG TGG TCG ACG GCC CGG GCT GGT-3′; and ADPR2: 3′-H2N-CCC GAC CA-PO4-5. The primers used are listed as follows – AP1: 5′-GTA ATA CGA CTC ACT ATA GGG C-3′; AP2: 5′-ACT ATA GGG CAC GCG TGG T-3′; LB1: 5′-GAC TCT AGC TAG AGT CAA GCA GAT CGT-3′; and LB2: 5′-GAT CGA CCG GCA TGC AAG-3′.
Cloning the full-length AtMSH5 cDNA
Full-length AtMSH5 cDNA was cloned according to the GeneRcer™ Kit (Invitrogen). In brief, 5 μg of the total bud RNA of the wild type was treated with calf intestinal phosphatase (CIP) to remove the 5′ phosphates, then the dephosphorylated RNA was treated with tobacco acid pyrophosphatase (TAP) to remove the 5′ cap structure from intact full-length mRNA. The GeneRacer™ RNA oligomers (5′-CGA CUG GAG CAC GAG GAC ACU GAC AUG GAC UGA AGG AGU AGA AA-3′) were then ligated to the 5′ end of the mRNA using T4 RNA ligase. Reverse transcription was carried out with the RNA oligomer-added mRNA, using AMVRT and the GeneRacer™ oligo-dT primer (5′-GCT GTC AAC GAT ACG CTA CGT AAC GGC ATG ACA GTG (T)18-3′), to create RACE-ready first-strand cDNA with known priming sites at the 5′ and 3′ ends. To obtain the 3′ ends, the PCR reaction was performed using the first strand of cDNA as a template with a forward gene-specific primer (GSP1: 5′-AGT GTT AGA TCG CCG TCT CAA TGC TA-3′) and the GeneRacerTM 3′ primer (5′-GCT GTC AAC GAT ACG CTA CGT AAC G-3′), and then nested PCR was carried out with a forward gene-specific nested primer (GSP2: 5′-GTC TAT GAA CTG GTC ATT GGA GTC A-3′) and the GeneRacerTM 3′ nested primer (5′-CGC TAC GTA ACG GCA TGA CAG TG-3′). To obtain the 5′ ends, the PCR reaction was performed using a reverse gene-specific primer (GSP3: 5′-CTG TCA GGA CAG GCC TTA CGT AGT T-3′) and the GeneRacerTM 5′ primer (5′-CGA CTG GAG CAC GAG GAC ACT GA-3′), followed by nested PCR with a reverse gene-specific nested primer (GSP4: 5′-GCC TTC AGC AGG TGA GCC GAG AAC A-3′) and the GeneRacerTM 5′ nested primer (5′-GGA CAC TGA CAT GGA CTG AAG GAG TA-3′).
Semi-quantitative RT-PCR for transcript expression
A First Strand DNA Synthesis Kit (TOYOBO) was used to synthesize cDNA from DNase I-treated total RNA that was extracted from Arabidopsis wild-type (Col) leaf, stem, bud, flower, and bud tissue from Atmsh5 mutant plants. Primers were designed to amplify a housekeeping gene TUBLIN 4 to equalize RNA loading into the RT-PCR reaction. These were TUB4F: 5′-CGA AAA CGC TGA CGA GTG TA-3′ and TUB4R: 5′-CCT TGG GAA TGG GAT AAG GT-3′. The AtMSH5 gene-specific primers were F1: 5′- GCA CTT GAC TGA GCT ACT TAA CGA GA-3′ and R1: 5′-TGA AAG AAG GCA TGG ATG TCA C-3′. The RT-PCR products were recovered and sequenced to confirm their authenticity and to exclude the possibility of RT-PCR template contamination by genomic DNA.
Characterization of mutant phenotypes
Plants were photographed with a Sony digital camera, DSC-W50 (Sony Corp., Tokyo, Japan). Pictures of the flower were taken using a Nikon SMZ1000 dissecting microscope (Nikon Corp., Tokyo, Japan). To determine pollen viability, mature anthers were stained with Alexander's staining solution 38 and photographed under an Olympus BX-51 microscope (Olympus, Tokyo, Japan). The confocal observation of ovules was performed according to the method described by Christensen et al. 39. Chromosome spreading and DAPI staining were performed as described previously 40. The number of chiasmata and the number of bivalents/univalents were counted according to the method described by Sanchez-Moran et al. 19.
Accession codes
References
Kolodner R . Biochemistry and genetics of eukaryotic mismatch repair. Genes Dev 1996; 10:1433–1442.
Modrich P . Mechanisms and biological effects of mismatch repair. Annu Rev Genet 1991; 25:229–253.
Chi NW, Kolodner RD . Purification and characterization of MSH1, a yeast mitochondrial protein that binds to DNA mismatches. J Biol Chem 1994; 269:29984–29992.
Reenan R, Kolodner RD . Characterization of insertion mutations in the Saccharomyces cerevisiae MSH1 and MSH2 genes: evidence for separate mitochondrial and nuclear functions. Genetics 1992; 132:975–985.
Hollingsworth NM, Ponte L, Halsey C . MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev 1995; 9:1728–1739.
Ross-Macdonald P, Roeder GS . Mutation of a meiosis-specific MutS homolog decreases crossing over but not mismatch correction. Cell 1994; 79:1069–1080.
Kelly KO, Dernburg AF, Stanfield GM, Villeneuve AM . Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 2000; 156:617–630.
de Vries SS, Baart EB, Dekker M, et al. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev 1999; 13:523–531.
Edelmann W, Cohen PE, Kneitz B, et al. Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet 1999; 21:123–127.
Bocker T, Barusevicius A, Snowden T, et al. hMSH5: A human MutS homologue that forms a novel heterodimer with hMSH4 and is expressed during spermatogenesis. Cancer Res 1999; 59:816–822.
Snowden T, Acharya S, Butz C, Berardini M, Fishel R . hMSH4-hMSH5 recognizes Holliday junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell 2004; 15:437–451.
Higgins JD, Armstrong SJ, Franklin FC, Jones GH . The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: evidence for two classes of recombination in Arabidopsis. Genes Dev 2004; 18:2557–2570.
Her C, Wu XL, Bailey SM, Doggett NA . Mouse MutS homolog 4 is predominantly expressed in testis and interacts with MutS homolog 5. Mammalian Genome 2001; 12:73–76.
Sekine H, Ferreira RC, Pan-Hammarström Q, et al. Role for Msh5 in the regulation of Ig class switch recombination. PNAS 2007; 104:7193–7198.
Sakamoto W, Kondo H, Murata M, Motoyoshi F . Altered mitochondrial gene expression in a maternal distorted leaf mutant of Arabidopsis lnduced by chloroplast mutator. Plant Cell 1996; 8:1377–1390.
Emmanuel E, Yehuda E, Melamed-Bessudo C, Avivi-Ragolsky N, Levy AA . The role of AtMSH2 in homologous recombination in Arabidopsis thaliana. EMBO Rep 2006; 1:100–105.
Hoffman PD, Leonard JM, Lindberg GE, Bollmann SR, Hays JB . Rapid accumulation of mutations during seed-to-seed propagation of mismatch-repair-defective Arabidopsis. Genes Dev 2004; 18:2676–2685.
Culligan KM, Hays JB . Arabidopsis MutS homologs—AtMSH2, AtMSH3, AtMSH6, and a Novel AtMSH7—form three distinct protein heterodimers with different specificities for mismatched DNA. Plant Cell 2000; 12:991–1002.
Sanchez Moran E, Armstrong SJ, Santos JL, Franklin FCH, Jones GH . Chiasma formation in Arabidopsis thaliana accession Wassileskija and in two meiotic mutants. Chromosome Res 2001; 9:121–128.
Biswas I, Hsieh P . Interaction of MutS protein with the major and minor grooves of a heteroduplex DNA. J Biol Chem 1997; 272:13355–13364.
Lamers MH, Perrakis A, Enzlin JH, Winterwerp HK, Wind Nd, Sixma TK . The crystal structure of DNA mismatch repair protein MutS binding to a G x T mismatch. Nature 2000; 407:711–717.
Obmolova G, Ban C, Hsieh P, Yang W . Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature 2000; 407:703–710.
Hsieh P . Molecular mechanisms of DNA mismatch repair. Mutat Res 2001; 4862:71–87.
Yang W, Steitz TA . The recently reported crystal structures of two recombination enzymes, the catalytic domain of HIV integrase and Escherichia coli RuvC, an endonuclease, are surprisingly similar to that of ribonuclease H suggesting the possibility that they have a common enzymatic mechanism. Structure 1995; 3:131–134.
Timsit Y . Convergent evolution of MutS and topoisomerase II for clamping DNA crossovers and stacked Holliday junctions. J Biomol Struct Dynamics 2001; 2:215–218.
Alani E, Sokolsky T, Studamire B, Miret JJ, Lahue RS . Genetic and biochemical analysis of Msh2p-Msh6p: role of ATP hydrolysis and Msh2p-Msh6p subunit interactions in mismatch base pair recognition. Mol Cell Biol 1997; 17:2436–2447.
Novak JE, Ross-Macdonald PB, Shirleen Roeder G . The budding yeast Msh4 protein functions in chromosome synapsis and the regulation of crossover distribution. Genetics 2001; 158:1013–1025.
Ross-Macdonald P, Shirleen Roeder G . Mutation of a meiosis- specific MutS homolog decreases crossing over but not mismatch correction. Cell 1994; 79:1069–1080.
Pochart P, Woltering D, Hollingsworth NM . Conserved properties between functionally distinct MutS homologs in yeast. J Biol Chem 1997; 272:30345–30349.
Jackson N, Sanchez-Moran E, Buckling E, Armstrong SJ, Jones GH, Franklin FCH . Reduced meiotic crossovers and delayed prophase I progression in AtMLH3-deficient Arabidopsis. EMBO J 2006; 25:1315–1323.
Zalevsky J, MacQueen AJ, Duffy JB, Kemphues KJ, Villeneuve AM . Crossing over during Caenorhabditis elegans meiosis requires a conserved MutS-based pathway that is partially dispensable in budding yeast. Genetics 1999; 153:1271–1283.
Santos Tdl, Hunter N, Lee C, Larkin B, Loidl J, Hollingsworth NM . The Mus81/Mms81 endonuclease acts independently of double Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics 2003; 164:81–94.
Copenhaver GP, Housworth EA, Stahl FW . Crossover interference in Arabidopsis. Genetics 2002; 160:1631–1639.
Mercier R, Jolivet S, Vezon D, et al. Two meiotic crossover classes cohabit in Arabidopsis: one is dependent on MER3, whereas the other one is not. Current Biology 2005; 15:692–701.
Chen Cb, Zhang W, Timofejeva L, Gerardin Yand, Ma H . The Arabidopsis ROCK-N-ROLLERS gene encodes a homolog of the yeast ATP-dependent DNA helicase MER3 and is required for normal meiotic crossover formation. Plant J 2005; 43:321–334.
Berchowitz LE, Francis KE, Bey AL, Copenhaver GP . The role of AtMUS81 in interference-insensitive crossovers in A. thaliana. PLoS Genet 2007; 3: e132.
Cottage A, Yang A, Maunders H, de Lacy RC, Ramsay NA . Identification of DNA sequences flanking T-DNA insertions by PCR-walking. Plant Mol Biol Rep 2001; 19:321–327.
Alexander MP . Differential staining of aborted and nonaborted pollen. Stain Technol 1969; 44:117–122.
Christensen CA, King EJ, Jordan JR, Drews GN . Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex Plant Reprod 1997; 10:49–64.
Ross KJ, Fransz P, Jones GH . A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome Res 1996; 4:507–516.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 30470173). The plant expression vector was kindly provided by Flanders Interuniversity Institute for Biotechnology (VIB), Belgium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, X., Liu, X., An, L. et al. The Arabidopsis MutS homolog AtMSH5 is required for normal meiosis. Cell Res 18, 589–599 (2008). https://doi.org/10.1038/cr.2008.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2008.44
Keywords
This article is cited by
-
Tracing the evolution of the plant meiotic molecular machinery
Plant Reproduction (2023)
-
Activation of C–C motif chemokine receptor 2 modulates testicular macrophages number, steroidogenesis, and spermatogenesis progression
Cell and Tissue Research (2021)
-
Involvement of DNA mismatch repair systems to create genetic diversity in plants for speed breeding programs
Plant Physiology Reports (2020)
-
Defects in cytoskeletal microtubule deployment of microsporocytes contribute to fertility loss in genic male-sterile Chinese cabbage
Plant Reproduction (2013)
-
Volume-based pollen size analysis: an advanced method to assess somatic and gametophytic ploidy in flowering plants
Plant Reproduction (2013)