ABSTRACT
Neurological complications associated with HIV-1/AIDS are being recognized with a high frequency that parallels the increased number of AIDS cases. The early infiltration by HIV-1 into the nervous system can cause primary and/or secondary neurological complications. The most common neurocognitive disorder is AIDS Dementia Complex (ADC). In developing countries of Asia the three most opportunistic infections are tuberculosis (TB), cryptococcosis, and Pneumocystis carinii pneumonia. Therefore, it is expected that secondary neurological complications due to TB and cryptococcosis will be the most common cause of morbility and mortality in HIV-1/AIDS cases in China. Research of NeuroAIDS in China is necessary to understand the impact and the biology of HIV-1 in the nervous system. Future studies would include, the molecular epidemiology and the description of opportunistic infections associated to HIV-1; the neuropathological description of primary and secondary HIV-1 complications in different groups; the HIV-1 neurotropism and immune response studies for China's unique HIV-1 strains and recombinant forms derived from the nervous system, including experimental models such as the use of transgenic rats; and the study of potential resistant virus, primarily when the anti-retroviral therapy (ART) has not full access in the brain.
Similar content being viewed by others
INTRODUCTION AND NEUROEPIDEMIOLOGY OF HIV/AIDS
AIDS was first recognized as a new and distinct clinical entity in 1981 1 and the HIV-1 as their casual agent in 1983 2. Since then, the HIV/AIDS epidemic has reached epidemic proportions with a total accumulative number of more than 60 million people, according to the Joint United Nations Programme on HIV/AIDS (UNAIDS) and WHO. The extensive spread of HIV-1 epidemics in Asia was not appreciated in the 1980s, however, several countries including China, India, Burma, Thailand, Indonesia and Vietnam have extremely growing epidemic 3. The first AIDS case in China was identified in Beijing in 1985 and since then the HIV-1 has spread rapidly through the whole country. By the end of 2002, more that 40, 000 HIV-1 cases were reported, and more than 2600 of cases were diagnosed with full-blown AIDS 4, 5. It is estimated that the present number of HIV-1 cases exceeds one million 6. By the year 2010 China is expected to reach 10 millions HIV-1 infected 7. The increase of prostitution, heterosexual transmission and injected drug users indicates the potential for HIV-1 spread in the future of China.
Neurological complications associated with HIV-1 are being recognized since the beginning of AIDS epidemic. The initial step in HIV-1 neuropathogenesis is viral entry into the central nervous system (CNS). This infiltration by HIV-1 can cause direct and/or indirect neurological complications 8. ADC is a common neurologic complication unique to HIV-1 infection 8. The prevalence of HIV-1-associated encephalitis is the sole manifestation in nearly 3–5% of patients with HIV-1 infection 9, with incidence ranging from 30 to 60% in the late stages of AIDS. In developing countries in Asia the three most opportunistic infections are TB, cryptococcosis, and Pneumocystis carinii pneumonia 10. Therefore, it is expected that secondary neurological complications due to TB and cryptococcosis will be the most common cause of morbility and mortality in HIV-1/AIDS cases in China. In addition, other frequent infections includes cytomegalovirus (CMV), toxoplasmosis and hepatitis C. Less common secondary neurological complications seen in patients with late stages of AIDS in developing countries includes JC virus associated progressive multifocal encephalopathy (PML) and HIV-1 associated malignancies primary CNS lymphoma. Overall in China, we expect HIV-1 associated neurological symptoms as a sole manifestation in nearly 50,000 of patients. In addition, we expect a high incidence of neurological complications ranging from 250,000 to more than half of a million of AIDS patients.
ART has clearly improved the morbidity and mortality in HIV-1 infected individuals by reducing plasma viral load and restoring immune function. Despite the overall improvement of outcome in those AIDS patients receiving ART, however, neurocognitive impairments continue to present in 10% of patients with HIV-1 associated dementia 11 and in up to 50% of patients with HIV-1 encephalopathy 12, 13. These findings could be attributed to AIDS patients developing resistance to or failure of ART 14, with the blood brain-barrier limiting access of anti-viral drugs to infected sites within the parenchyma 15. It is possible that cells from the CNS harbor latent HIV-1 and serve as a persistent reservoir of virus, responsible for viral reactivation and gradual neurocognitive decline.
NEUROTROPISM AND BRAIN COMPARTMENTALIZATION OF HIV-1
The HIV-1 is the agent that causes AIDS 2, and it is a RNA virus, which is a member of the retroviridae family. The genetic organization of HIV-1 is quite complex. In addition to the structural genes gag, pol and env possessed by all retroviruses, HIV-1 contains genes that encode for regulatory proteins: rev (regulator of virion structural protein), tat (trans-activator), and nef (negative regulatory factor); and genes that encode for proteins believed to be involved in virus maturation and release: vif (virion infectivity factor), vpu (virus protein U), and vpr (virus protein R). Based on relatedness of nucleotide sequences and the diversion seen among HIV-1 isolates from people of different countries, a classification of HIV-1 into subtypes or clades has been designed. Such subtypes, designed A through K, have envelope gene sequences that vary by 20% or more between subtypes.
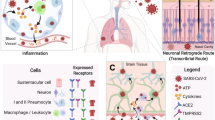
Several studies have demonstrated that microglia/macrophages are important cellular reservoirs for productive HIV-1 infection in the brain 10, 16, 17, 18, 19, 20, 21, 22. In addition, it has been shown that astrocytes can be infected by HIV-1 in vitro and in vivo, by the detection of early regulatory genes such as nef and rev 16, 18, 23, 24, 25. In general, the selectivity of HIV-1 strains for different cell types is regulated by interactions between the viral envelope and cellular receptors. For example, HIV-1 infection of T helper lymphocytes and monocytes/macrophages is dependent upon the presence of cellular CD4 receptors 26, 27. In addition, some members of the chemokine receptor family are required in conjunction with CD4 as coreceptors for HIV-1 entry into target cells; T-tropic HIV-1 utilizes CXCR4 as a co-receptor 28, whereas M-tropic HIV-1 utilizes CCR5 and CCR3 29, 30, 31, 32, 33, 34. HIV-1 can also infect other cell types that do not express CD4, including brain-derived glial 35 and neuronal cells 36, 37, human skin fibroblasts 38, muscle cells 39, human trophoblast cells 40, follicular dendritic cells 41, colonic epithelial cells 42, fetal adrenal cells 43, and human liver carcinoma cells 44.
The V3 loop of gp120 has been identified as a primary determinant of HIV-1 cell tropism 45, 46, 47. Sequences within the V3 loop has been shown to be associated with either the M-tropic, or T tropic phenotypes 45, 48, 49. We have demonstrated the importance of the V3 loop of T-tropic HIV-1 as a primary determinant for infectivity of CD4-negative neuronal SK-N-MC cells 50, and astrocytes 51. Collectively, these data indicate that infection and/or affinity of HIV-1 for cells of the nervous system, a phenomenon called neurotropism, is regulated by the interaction between viral epitopes (V3 loop) and the receptors (see Fig. 1).
Neuropathogenesis of HIV-1 Infection. Microglia infection by HIV-1 has been pathologically associated with “HIV-1 encephalitis”. The HIV-1 M tropic phenotype (V3 negative charges) is responsible for microglia infection in presence of CD4 receptor and CCR5 and CCR3 coreceptors. Neurons and astrocytes infection by HIV-1 has been associated with “HIV-1 encephalopathy”. The HIV-1 T tropic phenotype (V3 positive charges) is responsible for astrocyte/neuron infection in absence of CD4 receptor and some cases the presence of CXCR4.
The genetic evolution of HIV-1 within the brain is distinct from that in lymphoid tissues and other organs 52, 53. The genetic compartmentalization of viral variants in the CNS suggest that adaptive changes occur in response to unique constraints within the brain microenvironment, including specific target cell populations and immune selection pressures 54.
In one study, 37 full-length HIV-1 envelope glycoproteins (env) genes were cloned directly from brain biopsy and blood samples from patients with AIDS 55. Phylogenetic analysis showed distinct clustering of brain relative to blood env sequences, indicating tissue-specific compartmentalization of the virus. However, no brain-specific signature sequence was identified. Furthermore, there were no significant differences in the numbers or positions of N-linked glycosylation sites between brain and blood env sequences. The patterns of coreceptor usage were heterogeneous, with no clear distinction between brain and blood env clones. These results suggest that HIV-1 envelopes in brain cannot be distinguished from those in blood on the basis of coreceptor usage or the number or positions of N-glycosylation sites, indicating that other properties underlie neurotropism.
Astrocytes and microglia has been identified as target cells for HIV-1 infection in the brain, whereas most studies show that viral DNA is rarely detected in neurons 10, 16, 19, 56, 57. HIV-1 has been molecularly characterized from pure populations of astrocytes, macrophages and multinucleated giants cells and it was isolated from brain tissue of ADC patients 58. The V3 region of the HIV-1 env gene was amplified from the pure-cell populations, and multiple clones were sequenced. The V3 env sequences were distinct in astrocytes compared with neighboring macrophages or multinucleated giants cells and were characteristic of CCR5-using HIV-1. These results demonstrate cell-specific compartmentalization of distinct R5-like viral strains in the CNS microenvironment.
NERVOUS SYSTEM: LATENCY AND RESERVOIR FOR HIV-1
The capacity of HIV-1 in establishing latent infection of CD4+ T cells may allow viral persistence despite immune responses and antiretroviral therapy. Measurements of infectious virus, viral RNA in plasma, viral DNA, and viral messenger RNA species from infected cells are suggesting that HIV-1 replication continues throughout the course of infection. During the asymptomatic phase of infection subsist an extremely low total body load of latently infected resting CD4+ T cells with replication-competent integrated provirus (<107 cells). The most prevalent form of HIV-1 DNA, in resting and activated CD4+ T cells, is a full-length, linear, unintegrated form that is not replication competent. The infection progresses even though at any given time, the lymphoid tissues which has integrated HIV-1 DNA, is present in a minute fraction of susceptible populations, including resting and activated CD4+ T cells, and macrophages 59, 60. Furthermore, replication-competent virus has been recovered from resting CD4+ T lymphocytes in patients on ART. This reservoir of latent virus should be considered when deciding to terminate treatment in ART responder patients 61. Most anti-viral therapies used against HIV-1 collect poorly in the CNS because of efflux systems located at the blood-brain barrier (BBB), which rapidly return these drugs back to the circulation 62, 63, 64. As such, HIV-1 within the CNS may act as a reservoir for the re-infection of peripheral tissues.
Human monocytes play an important role in mediating HIV-1 infection of the CNS, and monocytes-derived macrophages represent a major viral reservoir within the brain and other target organs. Microglia are endogenous brain macrophages that show distinct phenotypes such as expression of myeloid antigens, ramified morphology, and location within the neural parenchyma. Microglia play a significant role in the developing of HIV-1-associated encephalitis. Together with monocyte-derived (perivascular) macrophages, microglia represent a major target of HIV-1 infection and these cells are considered traditionally the “brain reservoir” of the virus 65.
Alternatively, due to the large number of astrocytes (1:10) and its crucial role in the brain homeostasis, this cell type is believed to play a significant role in the development of HIV-1-associated encephalopathy. Astrocytes is also serving as brain reservoir for HIV-1. Infection of astrocytes by HIV-1 impairs its function directly and/or indirectly 66, 67. Lastly, multipotential human brain-derived progenitor cells and progenitor-derived astrocytes, but not progenitor-derived neurons, are permissive for HIV-1 and support a latent infection that can be reactivated by differentiation or cytokine stimulation. Progenitor cells in the brain therefore may represent an additional reservoir for HIV-1. These cells may be a source of infected and/or impaired astrocytes in pediatric cases of HIV-1 encephalopathy. These cells may also represent long-term targets for infection in adult patients, who are often living with the infection for 2 or 3 decades before signs of neurological damage are apparent 68.
Several studies have shown a large HIV-1 DNA load in the brains of some AIDS patients 69, 70. In the host cell, retroviral DNAs exist in three main forms: unintegrated linear, unintegrated circular, and integrated (the provirus). Each of the three species of viral DNA are detectable in blood and brain of AIDS patients. Autopsy samples from patients with HIV encephalitis had a considerable higher proportion of unintegrated viral DNA 71, 72. High levels of unintegrated forms of retroviral DNA often correlate with superinfection and accompanying cytopathic effects.
NEUROLOGICAL MANIFESTATIONS OF HIV-1/AIDS
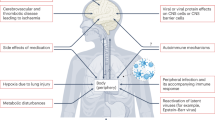
Neurological complications were recognized early in the AIDS epidemic as a consequence of HIV-1 infection. Neurological complications are detected in up to 60% of AIDS patients and are the result of direct (primary) HIV-1 infection and secondary (indirect) opportunistic infections and neoplasm 8 [See Fig. 2]. ADC or HIV-1-associated encephalitis is the most common neurological complication unique to HIV-1 infection 8. Neuropathological studies have suggested that more than 90% of all AIDS patients have nervous system abnormalities 8. These changes are primarily associated with opportunistic infections and neoplasms. Frequently, multiple, coexisting processes are found in the same patient 73. Neurological complications associated with HIV-1 infections vary geographically. Different profiles of neurological illness in AIDS patients are likely related to the geographic location of the reporting institution, the diagnostic studies available, and the percentage of patients from different groups, the time of stage of disease, time of analysis and the availability of ART.
Neurological Complications of HIV-1/AIDS. Primary or direct complication of HIV-1 infection includes ADC, myelopathy, peripheral neuropathy and myopathy. Secondary opportunistic infections includes viral (CMV), bacterial (TB), fungal (criptococcus), and parasitic (toxoplasmosis) infections. The most common HIV-1 associated malignancy is the primary non-Hodgkin's CNS lymphoma.
NeuroAIDS in Mexico and USA
To understand the pattern of HIV-1 associated neurological complications in Mexico in comparison with a population in USA, we conducted a cross-sectional and retrospective study in 120 AIDS patients from Mexico City, Mexico and 500 AIDS cases from Houston, Texas, USA 8. Neurological, laboratory, imaging and pathological examinations identified 40 Mexican and 130 USA patients with neurological complications. ADC was the most common complication in both groups, opportunistic infection such as intracranial tuberculoma was present only in the Mexican population. Tumors such as primary CNS lymphoma were more prevalent in the USA population. Similarly, PML occurred more commonly in the USA population. The different findings in the Mexican population likely reflect afflictions common to developing countries, a high prevalence of tuberculosis and a high mortality rate. These conditions preclude complication such as lymphoma and PML, which develop later in the natural course of HIV-1 infection. However, as HIV-1 patients from developing countries are living longer due to ART and prophylactic drugs for opportunistic infections, more common neurological complications are expected.
NeuroAIDS in Brazil
The first AIDS case in Brazil was reported in 1983. Since then, more than 360,000 cases of AIDS have been reported 74. One hospital which specializes in infectious disease, Institute Emilio Ribas, located in São Paulo, Brazil has admitted more than 30,000 AIDS patients in the last 15 years. Neurological complications have also critically impacted these patients. More than 2,000 patients with CNS cryptococcosis and almost 5,000 patients with CNS toxoplasmosis have been affected. At the Institute Emilio Ribas, a cohort study has been conducted investigating meningeal or meningoencephalitis syndrome. The most common causes for these syndromes were cryptococcosis, TB and syphilis. When brain expansive lesion syndrome predominates, the main causes included CNS toxoplasmosis, TB (tuberculomas or abscess), and primary CNS lymphoma 75. PML represents the most frequent cause of focal brain lesions without mass effect 76. The neurological complications of AIDS in this South American country highlight the importance of evaluating the neuroepidemiological setting in Brazil. For example, CNS TB represents the second most frequent cause of meningitis and expansive brain lesion 77, 78, 79. CNS toxoplasmosis, the most frequent CNS opportunistic disease, has been reduced to an incidence of 50 to 60% 80, which compares with a four-fold reduction in developed countries 81. In Brazil, CNS toxoplasmosis is considered as an AIDS-defining illness with severe immunodeficiency. CNS toxoplasmosis has a high mortality and disability rate, probably due to the epidemic characteristics of underdeveloped nations as heterosexual transmission, feminization and pauperization, and lack of ART 82. The prevalence of neurocognitives disorders and peripheral neuropathies in Brazilian AIDS patients has not being well defined. A molecular diagnosis of neurological opportunistic infections, referred to as “minimally invasive” will be part of a set of critical tools for prevalence analysis of international NeuroAIDS 83, 84. In summary, benefits of universal access programs to ART in Brazil are in some ways similar to developed countries, but there are urgent needs of a national network for surveillance, especially regarding incidence and the prevalence of neurological complications of HIV infection.
NeuroAIDS in India
India has the second largest burden of HIV related pathology next to sub-Saharan Africa. Neurological complications associated to HIV-1 infections, mainly clade C, are very common. The spectrum of HIV-1 associated complications reported within India (Bangalore in the south vs. Pune in the west) appears to be different. TB, cryptococcosis and toxoplasmosis are the major neuropathologies reflecting the endemicity and reactivation of latent infections 85. Viral infections and HIV-1 associated neoplasms such as primary CNS lymphoma are infrequent. ADC or HIV-1 associated encephalitis and myelopathies, are considered infrequent, though proper studies have just been initiated. Other HIV-1 associated complications including peripheral neuropathy have been reported. Future studies are important to understand the biology of neuroAIDS in India with its unique spectrum of opportunistic infections and HIV-1 clades.
FUTURE RESEARCH OF NEUROAIDS IN CHINA
Research of NeuroAIDS in China is essential to understand the impact and the biology of HIV-1 in the nervous system. Future studies would include, the molecular epidemiology and the description of opportunistic infections associated to HIV-1; the neuropathological description of primary and secondary HIV-1 complications in different high risk groups; the HIV-1 neurotropism and immune response studies for China's unique HIV-1 strains and recombinant forms derived from the nervous system, including experimental models such as the use of transgenic rats 86 to understand the HIV-1 neuropathogenesis of China; and the study of “potential” resistant virus, primarily when anti-retroviral therapy does not have full access in the brain. Latency, activation and reactivation of HIV-1 in the nervous system on HIV-1 in the brain are other major areas for future investigations, which will provide new insights into the development of therapeutic agents. If we can improve the neurological consequences of HIV-1/AIDS, we will improve the quality of life and the life expectancy of patients affected with this virus.
References
Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 1981; 305:1425–31.
Gallo RC, Sarin PS, Gelmann EP, et al. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 1983; 220:865–7.
Ruxrungtham K, Brown T, Phanuphak P . HIV/AIDS in Asia. Lancet 2004; 364:69–82.
Press office of Chinese Ministry of Health. The report of 2002 HIV/AIDS epidemic situation and the development of prevention & control in China. Beijing; February 2003.
Chinese Ministry of Health. The summary of HIV/AIDS of prevention and control in China (Chinese). Beijing; November 2002.
National Center for AIDS/STD Prevention and Control, Chinese Center for Disease Control and Prevention. Information on HIV/AIDS prevention and control. 2003; 12:212–5.
Center for Business and Government-Asia Programs. Kennedy School of Goverment, Harvard University. Novartis Foundation for Sustainable Development. Information for Health: The Harvard HIV/AIDS Initiative in China. December 2004.
Trujillo JR, Garcia-Ramos G, Novak IS, et al. Neurologic manifestations of AIDS: a comparative study of two populations from Mexico and the United States. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 8:23–9.
Janssen RS, Cornblath DR, Epstein LG, McArthur J, Price RW . Human immunodeficiency virus (HIV) infection and the nervous system: report from the American Academy of Neurology AIDS Task Force. Neurology 1989; 39:119–22.
An SF, Groves M, Giometto B, Beckett AA, Scaravilli F . Detection and localisation of HIV-1 DNA and RNA in fixed adult AIDS brain by polymerase chain reaction/in situ hybridisation technique. Acta Neuropathol (Berl) 1999; 98:481–7.
Dougherty RH, Skolasky RL Jr, McArthur JC . Progression of HIV-associated dementia treated with HAART. AIDS Read 2002; 12:69–74.
Lanska DJ . Epidemiology of human immunodeficiency virus infection and associated neurologic illness. Semin Neurol 1999; 19:105–11.
Masliah E, DeTeresa RM, Mallory ME, Hansen LA . Changes in pathological findings at autopsy in AIDS cases for the last 15 years. AIDS 2000; 14:69–74.
Letendre SL, McCutchan JA, Childers ME, et al. Predictors of improvement in human immunodefiency virus-associated neurocognitive disorders during antiretroviral therapy. Ann Neurol, in press.
Strain M, Letendre S, Pillai S, et al. Genetic Composition of HIV-1 in CSF and plasma without treatment and during failing combination antiretroviral therapy. J Virol, in press.
Bagasra O, Lavi E, Bobroski L, et al. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 1996; 10:573–85.
Glass JD, Fedor H, Wesselingh SL, McArthur JC . Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 1995; 38:755–62.
Nuovo GJ, Gallery F, MacConnell P, Braun A . In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acids and tumor necrosis factor-alpha RNA in the central nervous system. Am J Pathol 1994; 144:659–66.
Takahashi K, Wesselingh SL, Griffin DE, et al. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol 1996; 39:705–11.
Wang TH, Donaldson YK, Brettle RP, Bell JE, Simmonds P . Identification of shared populations of human immunodeficiency virus type 1 infecting microglia and tissue macrophages outside the central nervous system. J Virol 2001; 75:11686–99.
Wiley CA . Polymerase chain reaction in situ hybridization—opening Pandora's box? Ann Neurol 1996; 39:691–2.
Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB . Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA 1986; 83:7089–93.
Ranki A, Nyberg M, Ovod V, et al. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 1995; 9:1001–8.
Saito Y, Sharer LR, Epstein LG, et al. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology 1994; 44:474–81.
Tornatore C, Chandra R, Berger JR, Major EO . HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 1994; 44:481–7.
Klatzmann D, Champagne E, Chamaret S, et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 1984; 312:767–8.
Maddon PJ, Dalgleish AG, McDougal JS, et al. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 1986; 47:333–48.
Feng Y, Broder CC, Kennedy PE, Berger EA . HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996; 272:872–7.
Alkhatib G, Combadiere C, Broder CC, et al. CC-CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996; 272:1955–8.
Choe H, Farzan M, Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 1996; 85:1135–48.
Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996; 381:661–6.
Doranz B, Rucker JJ, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 1996; 85:1149–58.
Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996; 381:667–73.
He J, Chen Y, Farzan M, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 1997; 385:645–9.
Cheng-Mayer C, Rutka JT, Rosenblum ML, et al. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci USA 1987; 84:3526–30.
Harouse JM, Kunsch C, Hartle HT, et al. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol 1989; 63:2527–33.
Li XL, Moudgil T, Vinters HV, Ho DD . CD4-independent, productive infection of a neuronal cell line by human immunodeficiency virus type 1. J Virol 1990; 64:1383–7.
Tateno M, Gonzalez-Scarano F, Levy JA . Human immunodeficiency virus can infect CD4-negative human fibroblastoid cells. Proc Natl Acad Sci USA 1989; 86:4287–90.
Clapham PR, Weber JN, Whitby D, et al. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature 1989; 337:368–70.
Zachar V, Spire B, Hirsch I, Chermann JC, Ebbesen P . Human transformed trophoblast-derived cells lacking CD4 receptor exhibit restricted permissiveness for human immunodeficiency virus type 1. J Virol 1991; 65:2102–7.
Stahmer I, Zimmer JP, Ernst M, et al. Isolation of normal human follicular dendritic cells and CD4-independent in vitro infection by human immunodeficiency virus (HIV-1). Eur J Immunol 1991, 21:1873–8.
Yahi N, Baghdiguian S, Moreau H, Fantini J . Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol 1992; 66:4848–54.
Barboza A, Castro BA, Whalen M, et al. Infection of cultured human adrenal cells by different strains of HIV. AIDS 1992; 6:1437–43.
Cao YZ, Friedman-Kien AE, Huang YX, et al. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol 1990; 64:2553–9.
Hwang SS, Boyle TJ, Lyerly HK, Cullen BR . Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science 1991; 253:71–4.
O'Brien WA, Koyanagi Y, Namazie A, et al. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 1990; 348:69–73.
Shioda T, Levy JA, Cheng-Mayer C . Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature 1991; 349:167–9.
Chesebro B, Wehrly K, Nishio J, Perryman S . Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol 1992; 66:6547–54.
Kuiken CL, de Jong JJ, Baan E, et al. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol 1992; 66:4622–7.
Trujillo JR, Wang WK, Lee TH, Essex M . Identification of the envelope V3 loop as a determinant of a CD4-negative neuronal cell tropism for HIV-1. Virology 1996; 217:613–7.
Trujillo EB, Trujillo JR, Brain JD . The common molecular virology of HIV-1 tropism in “HIV-1 wasting syndrome” and “AIDS dementia complex”. Neurology 1997; 48:A94–5.
Hughes ES, Bell JE, Simmonds P . Investigation of the dynamics of the spread of human immunodeficiency virus to brain and other tissues by evolutionary analysis of sequences from the p17 gag and env genes. J Virol 1997; 71:1272–80.
Korber BTM, Kunstman KJ, Patterson BK, et al. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope glycoprotein of brain-derived sequences. J Virol 1994; 68:7467–81.
Shapshak P, Segal DM, Crandall KA, et al. Independent evolution of HIV type 1 in different brain regions. AIDS Res Hum Retroviruses 1999; 15:811–20.
Ohagen A, Devitt A, Kunstman KJ, et al. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol 2003; 77:12336–45.
Thompson KA, McArthur JC, Wesselingh SL . Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann Neurol 2001; 49:745–52.
Kaul M, Garden GA, Lipton SA . Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001; 410:988–94.
Thompson KA, Churchill MJ, Gorry PR, et al. Astrocyte specific viral strains in HIV dementia. Ann Neurol 2004; 56:873–7.
Chun TW, Finzi D, Margolick J, et al. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nature Med 1995; 1:1284–90.
Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997; 387:183–8.
Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300.
Glynn SL, Yazdanian M . In vitro blood-brain barrier permeability of nevirapine compared to other HIV antiretroviral agents. J Pharm Sci 1998; 87:306–10.
Lee CG, Gottesman MM, Cardarelli CO, et al. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 1998; 37:3594–601.
Masereeuw R, Jaehde U, Langemeijer MW, de Boer AG, Breimer DD . In vitro and in vivo transport of zidovudine (AZT) across the blood-brain barrier and the effect of transport inhibitors. Pharm Res 1994; 11:324–30.
Cosenza MA, Zhao ML, Si Q, Lee SC . Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol 2003; 12:442–55.
Brack-Werner R . Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 1999; 13:1–22.
Sabri F, Titanji K, De Milito A, Chiodi F . Astrocyte activation and apoptosis: their roles in the neuropathology of HIV infection. Brain Pathol 2003; 13:84–94.
Lawrence DM, Durham LC, Schwartz L, et al. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol 2004; 78:7319–28.
Bell JE, Busuttil A, Ironside JW, et al. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J Infect Dis 1993; 168:818–24.
Boni J, Emmerich BS, Leib SL, et al. PCR identification of HIV-1 DNA sequences in brain tissue of patients with AIDS encephalopathy. Neurology 1993; 43: 1813–7.
Pang S, Koyanagi Y, Miles S, et al. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature 1990; 343:85–9.
Teo I, Veryard C, Barnes H, et al. Circular forms of unintegrated human immunodeficiency virus type 1 DNA and high levels of viral protein expression: association with dementia and multinucleated giant cells in the brains of patients with AIDS. J Virol 1997; 71:2928–33.
Gildenberg PL, Langford L, Kim JH, Trujillo R . Stereotactic biopsy in cerebral lesions if AIDS. Acta Neurochir 1993; 58:68–70.
Ministério da Saúde do Brazil. 2005. Dados e Pesquisas em DST e AIDS. www.aids.gov.br
Madalosso G, Pellini AC, Vasconcelos MJ, et al. Chagasic meningoencephalitis: case report of a recently included AIDS-defining illness in Brazil. Rev Inst Med Trop Sao Paulo 2004; 46:199–202.
Fink MC, Penalva de Oliveira AC, Milagres FA, Vidal JE, Pannuti C . JC vírus DNA in cerebrospinal fluid samples from Brazilian aids patients with focal brain lesions without mass effect J Infect, in press.
Vidal JE, Oliveira AC, Dauar RF . Cerebral tuberculomas or tuberculous brain abscess: the dilemma continues. Clin Infect Dis 2005; 40:1072.
Vidal JE, Oliveira AC, Leite AG, et al. Tuberculous brain abscess in AIDS patients: report of three cases and literature review. Int J Infect Dis 2005; 9:201–7.
Vidal JE, Hernández AV, Oliveria AC, et al. Cerebral tuberculomas in AIDS patients: a forgotten diagnosis?. Arq Neuropsiquiatr 2004; 62:793–6.
Guimarães M . Temporal study in AIDS-associated disease in Brazil, 1980–1999. Cad Saude Publica 2000; 16:21–36.
Abgrall S, Rabaud C, Costagliola D, Clinical Epidemiology Group of the French Hospital Database on HIV. Incidence and risk factors for toxoplasmic encephalitis in human imunodeficiency virus-infected patients before and during the highly active antiretroviral therapy era. Clin Infect Dis 2001; 33:1747–55.
Vidal JE, Hernandez AV, de Oliveira AC, et al. Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS 2005; 19:626–34.
Vidal JE, Colombo FA, de Oliveira AC, Focaccia R, Pereira-Chioccola VL . PCR assay using cerebrospinal fluid for diagnosis of cerebral toxoplasmosis in Brazilian AIDS patients. J Clin Microbiol 2004; 42:4765–8.
Colombo FA, Vidal JE, Penalva de Oliveira AC, et al. Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: importance of molecular and immunological methods using peripheral blood samples. J Clin Microbiol 2005; 43:5044–7.
Shankar SK, Mahadevan A, Satishchandra P, et al. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J Med Res 2005; 121:468–88.
Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A 2001; 98:9271–9276.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
TRUJILLO, J., JARAMILLO-RANGEL, G., ORTEGA-MARTINEZ, M. et al. International NeuroAIDS: prospects of HIV-1 associated neurological complications. Cell Res 15, 962–969 (2005). https://doi.org/10.1038/sj.cr.7290374
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290374
Keywords
This article is cited by
-
Understanding HIV-associated neurocognitive and neurodegenerative disorders (neuroAIDS): enroute to achieve the 95-95-95 target and sustainable development goal for HIV/AIDS response
VirusDisease (2023)
-
Enhancement of NMDA Receptor-Mediated Excitatory Postsynaptic Currents by gp120-Treated Macrophages: Implications for HIV-1-Associated Neuropathology
Journal of Neuroimmune Pharmacology (2013)
-
Tight Junction Regulation by Morphine and HIV-1 Tat Modulates Blood–Brain Barrier Permeability
Journal of Clinical Immunology (2008)
-
BK virus associated meningoencephalitis in an AIDS patient treated with HAART
AIDS Research and Therapy (2007)