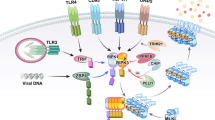
Abstract
Receptor-interacting protein (RIP) kinases are a group of threonine/serine protein kinases with a relatively conserved kinase domain but distinct non-kinase regions. A number of different domain structures, such as death and caspase activation and recruitment domain (CARD) domains, were found in different RIP family members, and these domains should be keys in determining the specific function of each RIP kinase. It is known that RIP kinases participate in different biological processes, including those in innate immunity, but their downstream substrates are largely unknown. This review will give an overview of the structures and functions of RIP family members, and an update of recent progress in RIP kinase research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P . RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ 2007; 14: 400–410.
Declercq W, Vanden Berghe T, Vandenabeele P . RIP kinases at the crossroads of cell death and survival. Cell 2009; 138: 229–232.
Meylan E, Tschopp J . The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci 2005; 30: 151–159.
Korr D, Toschi L, Donner P, Pohlenz HD, Kreft B, Weiss B . LRRK1 protein kinase activity is stimulated upon binding of GTP to its Roc domain. Cell Signal 2006; 18: 910–920.
Stanger BZ, Leder P, Lee TH, Kim E, Seed B . RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 1995; 81: 513–523.
Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L . Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 1997; 7: 821–830.
Wen L, Zhuang L, Luo X, Wei P . TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem 2003; 278: 39251–39258.
Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV . TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 1996; 4: 387–396.
Varfolomeev EE, Boldin MP, Goncharov TM, Wallach D . A potential mechanism of “cross-talk” between the p55 tumor necrosis factor receptor and Fas/APO1: proteins binding to the death domains of the two receptors also bind to each other. J Exp Med 1996; 183: 1271–1275.
Ahmad M, Srinivasula SM, Wang L, Talanian RV, Litwack G, Fernandes-Alnemri T et al. CRADD, a novel human apoptotic adaptor molecule for caspase-2, and FasL/tumor necrosis factor receptor-interacting protein RIP. Cancer Res 1997; 57: 615–619.
Duan H, Dixit VM . RAIDD is a new ‘death’ adaptor molecule. Nature 1997; 385: 86–89.
Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S et al. Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res 2000; 254: 14–24.
Bradley JR, Pober JS . Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 2001; 20: 6482–6491.
Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol 2004; 5: 503–507.
Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004; 430: 694–699.
Kim JW, Joe CO, Choi EJ . Role of receptor-interacting protein in tumor necrosis factor-alpha -dependent MEKK1 activation. J Biol Chem 2001; 276: 27064–27070.
Kurenova E, Xu LH, Yang X, Baldwin AS Jr, Craven RJ, Hanks SK et al. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol 2004; 24: 4361–4371.
Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM . RIP3, a novel apoptosis-inducing kinase. J Biol Chem 1999; 274: 16871–16875.
Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L et al. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol 2001; 2: 620–624.
Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol 1999; 9: 539–542.
Lee TH, Shank J, Cusson N, Kelliher MA . The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem 2004; 279: 33185–33191.
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P . The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998; 8: 297–303.
Micheau O, Tschopp J . Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003; 114: 181–190.
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr . NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998; 281: 1680–1683.
Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J . NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 2001; 21: 5299–5305.
Zhang H, Lin Y, Li J, Pober JS, Min W . RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation. J Biol Chem 2007; 282: 14788–14796.
Hsu H, Shu HB, Pan MG, Goeddel DV . TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996; 84: 299–308.
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137: 1112–1123.
He S, Wang L, Miao L, Wang T, Du F, Zhao L et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009; 137: 1100–1111.
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325: 332–336.
Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM . Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem 2002; 277: 9505–9511.
Kuang AA, Diehl GE, Zhang J, Winoto A . FADD is required for DR4- and DR5-mediated apoptosis: lack of trail-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J Biol Chem 2000; 275: 25065–25068.
Lawrence CP, Chow SC . FADD deficiency sensitises Jurkat T cells to TNF-alpha-dependent necrosis during activation-induced cell death. FEBS Lett 2005; 579: 6465–6472.
Wilson NS, Dixit V, Ashkenazi A . Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol 2009; 10: 348–355.
Inohara N, del Peso L, Koseki T, Chen S, Nunez G . RICK, a novel protein kinase containing a caspase recruitment domain, interacts with CLARP and regulates CD95-mediated apoptosis. J Biol Chem 1998; 273: 12296–12300.
McCarthy JV, Ni J, Dixit VM . RIP2 is a novel NF-kappaB-activating and cell death-inducing kinase. J Biol Chem 1998; 273: 16968–16975.
Thome M, Hofmann K, Burns K, Martinon F, Bodmer JL, Mattmann C et al. Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr Biol 1998; 8: 885–888.
Navas TA, Baldwin DT, Stewart TA . RIP2 is a Raf1-activated mitogen-activated protein kinase kinase. J Biol Chem 1999; 274: 33684–33690.
Jacquet S, Nishino Y, Kumphune S, Sicard P, Clark JE, Kobayashi KS et al. The role of RIP2 in p38 MAPK activation in the stressed heart. J Biol Chem 2008; 283: 11964–11971.
Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G . Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 2002; 416: 190–194.
Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 2002; 416: 194–199.
Inohara N, Nunez G . NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol 2003; 3: 371–382.
Viala J, Sansonetti P, Philpott DJ . Nods and ‘intracellular’ innate immunity. C R Biol 2004; 327: 551–555.
Nembrini C, Kisielow J, Shamshiev AT, Tortola L, Coyle AJ, Kopf M et al. The kinase activity of Rip2 determines its stability and consequently Nod1- and Nod2-mediated immune responses. J Biol Chem 2009; 284: 19183–19188.
Krieg A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huang Z et al. XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci USA 2009; 106: 14524–14529.
Li L, Bin LH, Li F, Liu Y, Chen D, Zhai Z et al. TRIP6 is a RIP2-associated common signaling component of multiple NF-kappaB activation pathways. J Cell Sci 2005; 118: 555–563.
Clark NM, Marinis JM, Cobb BA, Abbott DW . MEKK4 sequesters RIP2 to dictate NOD2 signal specificity. Curr Biol 2008; 18: 1402–1408.
Tao M, Scacheri PC, Marinis JM, Harhaj EW, Matesic LE, Abbott DW . ITCH K63-ubiquitinates the NOD2 binding protein, RIP2, to influence inflammatory signaling pathways. Curr Biol 2009; 19: 1255–1263.
Martinon F, Tschopp J . Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004; 117: 561–574.
Krieg A, Le Negrate G, Reed JC . RIP2-beta: a novel alternative mRNA splice variant of the receptor interacting protein kinase RIP2. Mol Immunol 2009; 46: 1163–1170.
Yin X, Krikorian P, Logan T, Csizmadia V . Induction of RIP-2 kinase by proinflammatory cytokines is mediated via NF-kappaB signaling pathways and involves a novel feed-forward regulatory mechanism. Mol Cell Biochem; 333: 251–259.
Pazdernik NJ, Donner DB, Goebl MG, Harrington MA . Mouse receptor interacting protein 3 does not contain a caspase-recruiting or a death domain but induces apoptosis and activates NF-kappaB. Mol Cell Biol 1999; 19: 6500–6508.
Feng S, Ma L, Yang Y, Wu M . Truncated RIP3 (tRIP3) acts upstream of FADD to induce apoptosis in the human hepatocellular carcinoma cell line QGY-7703. Biochem Biophys Res Commun 2006; 347: 558–565.
Newton K, Sun X, Dixit VM . Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 2004; 24: 1464–1469.
Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 2007; 19: 2056–2067.
Bhr C, Rohwer A, Stempka L, Rincke G, Marks F, Gschwendt M . DIK, a novel protein kinase that interacts with protein kinase Cdelta. Cloning, characterization, and gene analysis. J Biol Chem 2000; 275: 36350–36357.
Chen L, Haider K, Ponda M, Cariappa A, Rowitch D, Pillai S . Protein kinase C-associated kinase (PKK), a novel membrane-associated, ankyrin repeat-containing protein kinase. J Biol Chem 2001; 276: 21737–21744.
Holland P, Willis C, Kanaly S, Glaccum M, Warren A, Charrier K et al. RIP4 is an ankyrin repeat-containing kinase essential for keratinocyte differentiation. Curr Biol 2002; 12: 1424–1428.
Meylan E, Martinon F, Thome M, Gschwendt M, Tschopp J . RIP4 (DIK/PKK), a novel member of the RIP kinase family, activates NF-kappa B and is processed during apoptosis. EMBO Rep 2002; 3: 1201–1208.
Muto A, Ruland J, McAllister-Lucas LM, Lucas PC, Yamaoka S, Chen FF et al. Protein kinase C-associated kinase (PKK) mediates Bcl10-independent NF-kappa B activation induced by phorbol ester. J Biol Chem 2002; 277: 31871–31876.
Moran ST, Haider K, Ow Y, Milton P, Chen L, Pillai S . Protein kinase C-associated kinase can activate NFkappaB in both a kinase-dependent and a kinase-independent manner. J Biol Chem 2003; 278: 21526–21533.
Cariappa A, Chen L, Haider K, Tang M, Nebelitskiy E, Moran ST et al. A catalytically inactive form of protein kinase C-associated kinase/receptor interacting protein 4, a protein kinase C beta-associated kinase that mediates NF-kappa B activation, interferes with early B cell development. J Immunol 2003; 171: 1875–1880.
Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science 1999; 284: 316–320.
Li Q, Lu Q, Hwang JY, Buscher D, Lee KF, Izpisua-Belmonte JC et al. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev 1999; 13: 1322–1328.
Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T et al. Limb and skin abnormalities in mice lacking IKKalpha. Science 1999; 284: 313–316.
Zha J, Zhou Q, Xu LG, Chen D, Li L, Zhai Z et al. RIP5 is a RIP-homologous inducer of cell death. Biochem Biophys Res Commun 2004; 319: 298–303.
Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 2004; 44: 595–600.
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004; 44: 601–607.
Greggio E, Lewis PA, van der Brug MP, Ahmad R, Kaganovich A, Ding J et al. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J Neurochem 2007; 102: 93–102.
Haugarvoll K, Toft M, Ross OA, White LR, Aasly JO, Farrer MJ . Variants in the LRRK1 gene and susceptibility to Parkinson's disease in Norway. Neurosci Lett 2007; 416: 299–301.
Elbaz A . LRRK2: bridging the gap between sporadic and hereditary Parkinson's disease. Lancet Neurol 2008; 7: 562–564.
Anand VS, Braithwaite SP . LRRK2 in Parkinson's disease: biochemical functions. FEBS J 2009; 276: 6428–6435.
Paisan-Ruiz C . LRRK2 gene variation and its contribution to Parkinson disease. Hum Mutat 2009; 30: 1153–1160.
Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 2006; 23: 329–341.
Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA . Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 2006; 9: 1231–1233.
Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z . Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J Neurochem 2007; 103: 238–247.
Lewis PA, Greggio E, Beilina A, Jain S, Baker A, Cookson MR . The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun 2007; 357: 668–671.
Jorgensen ND, Peng Y, Ho CC, Rideout HJ, Petrey D, Liu P et al. The WD40 Domain Is Required for LRRK2 Neurotoxicity. PLoS One 2009; 4: e8463.
Benamer HT . The ancestry of LRRK2 Gly2019Ser parkinsonism. Lancet Neurol 2008; 7: 769–770; author reply 770–761.
Braithwaite SP . LRRK2 in Parkinson's disease: building an understanding of disease etiology. FEBS J 2009; 276: 6427.
Webber PJ, West AB . LRRK2 in Parkinson's disease: function in cells and neurodegeneration. FEBS J 2009; 276: 6436–6444.
Johansen KK, Wang L, Aasly JO, White LR, Matson WR, Henchcliffe C et al. Metabolomic profiling in LRRK2-related Parkinson's disease. PLoS One 2009; 4: e7551.
Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci 2009; 12: 826–828.
Ding X, Goldberg MS . Regulation of LRRK2 stability by the E3 ubiquitin ligase CHIP. PLoS One 2009; 4: e5949.
Gloeckner CJ, Schumacher A, Boldt K, Ueffing M . The Parkinson disease-associated protein kinase LRRK2 exhibits MAPKKK activity and phosphorylates MKK3/6 and MKK4/7, in vitro. J Neurochem 2009; 109: 959–968.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
This article is cited by
-
Screening of hub inflammatory bowel disease biomarkers and identification of immune-related functions based on basement membrane genes
European Journal of Medical Research (2023)
-
Different types of cell death and their shift in shaping disease
Cell Death Discovery (2023)
-
Functions of the RIP kinase family members in the skin
Cellular and Molecular Life Sciences (2023)
-
Sirt6 protects cardiomyocytes against doxorubicin-induced cardiotoxicity by inhibiting P53/Fas-dependent cell death and augmenting endogenous antioxidant defense mechanisms
Cell Biology and Toxicology (2023)
-
Synthesis of [11C]carbonyl-labeled cyclohexyl (5-(2-acetamidobenzo[d]thiazol-6-yl)-2-methylpyridin-3-yl)carbamate ([11C-carbonyl]PK68) as a potential PET tracer for receptor-interacting protein 1 kinase
EJNMMI Radiopharmacy and Chemistry (2022)