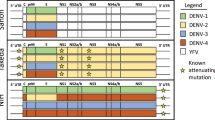
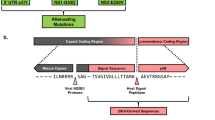
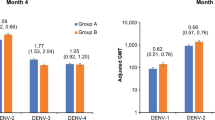
Abstract
Flaviviruses comprise approximately 70 closely related RNA viruses. These include several mosquito-borne pathogens, such as yellow fever virus (YFV), dengue virus (DENV), and Japanese encephalitis virus (JEV), which can cause significant human diseases and thus are of great medical importance. Vaccines against both YFV and JEV have been used successfully in humans for decades; however, the development of a DENV vaccine has encountered considerable obstacles. Here, we review the protective immune responses elicited by the vaccine against YFV to provide some insights into the development of a protective DENV vaccine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mackenzie JS, Gubler DJ, Petersen LR . Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 2004; 10: S98–S109.
World Health Organization. Yellow fever fact sheet No.100. [Updated March 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs100/en/#.
World Health Organization. Japanese encephalitis fact sheet No. 386. [Updated March 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs386/en/.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL et al. The global distribution and burden of dengue. Nature 2013; 496: 504–507.
Kramer LD, Ebel GD . Dynamics of flavivirus infection in mosquitoes. Adv Virus Res 2003; 60: 187–232.
Aitken TH, Tesh RB, Beaty BJ, Rosen L . Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti). Am J Trop Med Hyg 1979; 28: 119–121.
Rosen L, Tesh RB, Lien JC, Cross JH . Transovarial transmission of Japanese encephalitis virus by mosquitoes. Science 1978; 199: 909–911.
Chambers TJ, Hahn CS, Galler R, Rice CM . Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 1990; 44: 649–688.
Rice CM, Lenches EM, Eddy SR, Shin SJ, Sheets RL, Strauss JH . Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science 1985; 229: 726–733.
Modis Y, Ogata S, Clements D, Harrison SC . Structure of the dengue virus envelope protein after membrane fusion. Nature 2004; 427: 313–319.
Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002; 108: 717–725.
Murphy BR, Whitehead SS . Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 2011; 29: 587–619.
Mackenzie JM, Jones MK, Westaway EG . Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J Virol 1999; 73: 9555–9567.
Chu PW, Westaway EG . Replication strategy of Kunjin virus: evidence for recycling role of replicative form RNA as template in semiconservative and asymmetric replication. Virol 1985; 140: 68–79.
Coia G, Parker MD, Speight G, Byrne ME, Westaway EG . Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol 1988; 69: 1–21.
Mackenzie JM, Khromykh AA, Jones MK, Westaway EG . Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virol 1998; 245: 203–215.
Westaway EG, Khromykh AA, Kenney MT, Mackenzie JM, Jones MK . Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virol 1997; 234: 31–41.
Westaway EG, Mackenzie JM, Kenney MT, Jones MK, Khromykh AA . Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol 1997; 71: 6650–6661.
Khromykh AA, Varnavski AN, Sedlak PL, Westaway EG . Coupling between replication and packaging of flavivirus RNA: evidence derived from the use of DNA-based full-length cDNA clones of Kunjin virus. J Virol 2001; 75: 4633–4640.
Macnamara FN . Reactions following neurotropic yellow fever vaccine given by scarification in Nigeria. Trans R Soc Trop Med Hyg 1953; 47: 199–208.
Theiler M, Smith HH . The effect of prolonged cultivation in vitro upon the pathogenicity of yellow fever virus. J Exp Med 1937; 65: 767–786.
Theiler M, Smith HH . The use of yellow fever virus modified by in vitro cultivation for human immunization. J Exp Med 1937; 65: 787–800.
Pulendran B . Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat Rev Immunol 2009; 9: 741–747.
Pulendran B, Oh JZ, Nakaya HI, Ravindran R, Kazmin DA . Immunity to viruses: learning from successful human vaccines. Immunol Rev 2013; 255: 243–255.
Reinhardt B, Jaspert R, Niedrig M, Kostner C, L’age-Stehr J . Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol 1998; 56: 159–167.
Edupuganti S, Eidex RB, Keyserling H, Akondy RS, Lanciotti R, Orenstein W et al. A randomized, double-blind, controlled trial of the 17D yellow fever virus vaccine given in combination with immune globulin or placebo: comparative viremia and immunogenicity. Am J Trop Med Hyg 2013; 88: 172–177.
Albert BS . Research on dengue during World War II. Arch Virol 1952; 1: 30–50.
Luiza-Silva M, Campi-Azevedo AC, Batista MA, Martins MA, Avelar RS, Da Silveira Lemos D et al. Cytokine signatures of innate and adaptive immunity in 17DD yellow fever vaccinated children and its association with the level of neutralizing antibody. J Infect Dis 2011; 204: 873–883.
Banchereau J, Steinman RM . Dendritic cells and the control of immunity. Nature 1998; 392: 245–252.
Steinman R, Banchereau J . Taking dendritic cells into medicine. Nature 2007; 449: 419–426.
Pulendran B . Variegation of the immune response with dendritic cells and pathogen recognition receptors. J Immunol 2005; 174: 2457–2465.
Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 2009; 10: 116–125.
Barba-Spaeth G, Longman RS, Albert ML, Rice CM . Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J Exp Med 2005; 202: 1179–1184.
Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 2006; 203: 413–424.
Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R . Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2001; 2: 947–950.
Dillon S, Agrawal A, Van Dyke T, Landreth G, Mccauley L, Koh A et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated proteinkinase and c-Fos in dendritic cells. J Immunol 2004; 172: 4733–4743.
Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol 2003; 171: 4984–4989.
Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 2008; 205: 3119–3131.
Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L . NK cells at the interface between innate and adaptive immunity. Cell Death Differ 2008; 15: 226–233.
Neves PC, Matos DC, Marcovistz R, Galler R . TLR expression and NK cell activation after human yellow fever vaccination. Vaccine 2009; 27: 5543–5549.
Silva ML, Martins MA, Espirito-Santo LR, Campi-Azevedo AC, Silveira-Lemos D, Ribeiro JG et al. Characterization of main cytokine sources from the innate and adaptive immune responses following primary 17DD yellow fever vaccination in adults. Vaccine 2011; 29: 583–592.
Liprandi F, Walder R . Replication of virulent and attenuated strains of yellow fever virus in human monocytes and macrophage-like cells (U937). Arch Virol 1983; 76: 51–61.
Martins MA, Silva ML, Eloi-Santos SM, Ribeiro JG, Peruhype-Magalhaes V, Marciano AP et al. Innate immunity phenotypic features point toward simultaneous raise of activation and modulation events following 17DD live attenuated yellow fever first-time vaccination. Vaccine 2008; 26: 1173–1184.
Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 2002; 168: 3536–3542.
Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol 2009; 183: 7919–7930.
De Melo AB, Nascimento EJ, Braga-Neto U, Dhalia R, Silva AM, Oelke M et al. T-cell memory responses elicited by yellow fever vaccine are targeted to overlapping epitopes containing multiple HLA-I and -II binding motifs. PLoS Negl Trop Dis 2013; 7: e1938.
Co MD, Terajima M, Cruz J, Ennis FA, Rothman AL . Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: identification of HLA-B35-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology 2002; 293: 151–163.
Co MD, Kilpatrick ED, Rothman AL . Dynamics of the CD8 T-cell response following yellow fever virus 17D immunization. Immunology 2009; 128: e718–e727.
Miller JD, Van Der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 2008; 28: 710–722.
Blom K, Braun M, Ivarsson MA, Gonzalez VD, Falconer K, Moll M et al. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J Immunol 2013; 190: 2150–2158.
Bassi MR, Kongsgaard M, Steffensen MA, Fenger C, Rasmussen M, Skjodt K et al. CD8+ T cells complement antibodies in protecting against yellow fever virus. J Immunol 2015; 194: 1141–1153.
Zellweger RM, Tang WW, Eddy WE, King K, Sanchez MC, Shresta S . CD8+ T cells can mediate short-term protection against heterotypic dengue virus reinfection in mice. J Virol 2015; 89: 6494–6505.
Santos AP, Matos DC, Bertho AL, Mendonca SC, Marcovistz R . Detection of Th1/Th2 cytokine signatures in yellow fever 17DD first-time vaccines through ELISpot assay. Cytokine 2008; 42: 152–155.
Kohler S, Bethke N, Böthe M, Sommerick S, Frentsch M, Romagnani C et al. The early cellular signatures of protective immunity induced by live viral vaccination. Eur J Immunol 2012; 42: 2363–2373.
James EA, Lafond RE, Gates TJ, Mai DT, Malhotra U, Kwok WW . Yellow fever vaccination elicits broad functional CD4+ T cell responses that recognize structural and nonstructural proteins. J Virol 2013; 87: 12794–12804.
Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13: 843–850.
Barrett AD, Teuwen DE . Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol 2009; 21: 308–313.
Muyanja E, Ssemaganda A, Ngauv P, Cubas R, Perrin H, Srinivasan D et al. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest 2014; 124: 3147–3158.
Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K . Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull World Health Org 1981; 59: 895–900.
Brandriss MW, Schlesinger JJ, Walsh EE, Briselli M . Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol 1986; 67: 229–234.
Schlesinger JJ, Brandriss MW, Cropp CB, Monath TP . Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J Virol 1986; 60: 1153–1155.
Thibodeaux BA, Garbino NC, Liss NM, Piper J, Schlesinger JJ, Blair CD et al. A humanized IgG but not IgM antibody is effective in prophylaxis and therapy of yellow fever infection in an AG129/17D-204 peripheral challenge mouse model. Antiviral Res 2012; 94: 1–8.
Monath TP, Vasconcelos PF . Yellow fever. J Clin Virol 2014; 64: 160–73
Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (Arilvax and YF-VAX) in a Phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg 2002; 66: 533–541.
Julander JG, Trent DW, Monath TP . Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine 2011; 29: 6008–6016.
Kay A, Chen LH, Sisti M, Monath TP . Short report: yellow fever vaccine seroconversion in travelers. Am J Trop Med Hyg 2011; 85: 748–749.
Hepburn MJ, Kortepeter MG, Pittman PR, Boudreau EF, Mangiafico JA, Buck PA et al. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine 2006; 24: 2843–2849.
Osei-Kwasi M, Dunyo SK, Koram KA, Afari EA, Odoom JK, Nkrumah FK . Antibody response to 17D yellow fever vaccine in Ghanaian infants. Bull World Health Org 2001; 79: 1056–1059.
Lang J, Zuckerman J, Clarke P, Barrett P, Kirkpatrick C, Blondeau C . Comparison of the immunogenicity and safety of two 17D yellow fever vaccines. Am J Trop Med Hyg 1999; 60: 1045–1050.
Groot H, Bahiarib. R . Neutralizing and haemagglutination-inhibiting antibodies to yellow fever 17 years after vaccination with 17D vaccine. Bull World Health Org 1962; 27: 699–707.
Vaccines. CGFSOYF. Duration of post-vaccination immunity against yellow fever in adults. Vaccine 2014; 32: 4977–4984.
Gotuzzo E, Yactayo S, Cordova E . Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg 2013; 89: 434–444.
Niedrig M, Lademann M, Emmerich P, Lafrenz M . Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health 1999; 4: 867–871.
Monath TP . Neutralizing antibody responses in the major immunoglobulin classes to yellow fever 17D vaccination of humans. Am J Epidemiol 1971; 93: 122–129.
Gibney KB, Edupuganti S, Panella AJ, Kosoy OI, Delorey MJ, Lanciotti RS et al. Detection of anti-yellow fever virus immunoglobulin M antibodies at 3–4 years following yellow fever vaccination. Am J Trop Med Hyg 2012; 87: 1112–1115.
Heinz FX, Stiasny K . Flaviviruses and their antigenic structure. J Clin Virol 2012; 55: 289–295.
Monath TP . Yellow fever: an update. Lancet Infect Dis 2001; 1: 11–20.
Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med 2005; 11: 522–530.
Beasley DW, Barrett AD . Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J Virol 2002; 76: 13097–13100.
Sukupolvi-Petty S, Austin SK, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ et al. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J Virol 2007; 81: 12816–12826.
Vratskikh O, Stiasny K, Zlatkovic J, Tsouchnikas G, Jarmer J, Karrer U et al. Dissection of antibody specificities induced by yellow fever vaccination. PLoS Pathog 2013; 9: e1003458.
Schlesinger JJ, Brandriss MW, Walsh EE . Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein Gp48 and by active immunization with Gp48. J Immunol 1985; 135: 2805–2809.
Schlesinger JJ, Foltzer M, Chapman S . The Fc portion of antibody to yellow-fever virus-Ns1 is a determinant of protection against yellow fever encephalitis in mice. Virology 1993; 192: 132–141.
Pierson TC, Fremont DH, Kuhn RJ, Diamond MS . Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 2008; 4: 229–238.
Russell PK, Nisalak A . Dengue virus identification by the plaque reduction neutralization test. J Immunol 1967; 99: 291–296.
Normile D . Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science 2013; 342: 415.
Halstead SB . Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 2003; 60: 421–467.
Morens DM, Halstead SB . Measurement of antibody-dependent infection enhancement of four dengue virus serotypes by monoclonal and polyclonal antibodies. J Gen Virol 1990; 71 (Pt12): 2909–2914.
Guzman MG, Alvarez M, Halstead SB . Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 2013; 158: 1445–1459.
Osorio JE, Velez ID, Thomson C, Lopez L, Jimenez A, Haller AA et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14: 830–838.
Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis 2013; 207: 957–965.
Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine 2011; 29: 960–968.
Huang CY, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM . Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol 2003; 77: 11436–11447.
George SL, Wong MA, Dube TJ, Boroughs KL, Stovall JL, Luy BE et al. Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded Phase 1 clinical trial. J Infect Dis 2015; doi:10.1093/infdis/jiv179
Blaney JE, Durbin AP, Murphy BR, Whitehead SS . Development of a live attenuated dengue virus vaccine using reverse genetics. Viral Immunol 2006; 19: 10–32.
Durbin AP, Mcarthur JH, Marron JA, Blaney JE, Thumar B, Wanionek K et al. rDEN2/4Delta30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naïve adults. Hum Vaccin 2006; 2: 255–260.
Kirkpatrick BD, Durbin AP, Pierce KK, Carmolli MP, Tibery CM, Grier PL et al. Robust and balanced immune responses to all 4 dengue virus serotypes following administration of a single dose of a live attenuated tetravalent dengue vaccine to healthy, flavivirus-naive adults. J Infect Dis 2015; 212 (5):702–710.
Kanesa-Thasan N, Edelman R, Tacket CO, Wasserman SS, Vaughn DW, Coster TS et al. Phase 1 studies of Walter Reed Army Institute of Research candidate attenuated dengue vaccines: selection of safe and immunogenic monovalent vaccines. Am J Trop Med Hyg 2003; 69: 17–23.
Simasathien S, Thomas SJ, Watanaveeradej V, Nisalak A, Barberousse C, Innis BL et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus naive children. Am J Trop Med Hyg 2008; 78: 426–433.
Watanaveeradej V, Simasathien S, Nisalak A, Endy TP, Jarman RG, Innis BL et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus-naive infants. Am J Trop Med Hyg 2011; 85: 341–351.
Watanaveeradej V, Gibbons RV, Simasathien S, Nisalak A, Jarman RG, Kerdpanich A et al. Safety and immunogenicity of a rederived, live-attenuated dengue virus vaccine in healthy adults living in Thailand: a randomized trial. Am J Trop Med Hyg 2014; 91: 119–128.
Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHJM, Chotpitayasunondh T, Chua MN et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384: 1358–1365.
Guirakhoo F, Weltzin R, Chambers TJ, Zhang ZX, Soike K, Ratterree M et al. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol 2000; 74: 5477–5485.
Guirakhoo F, Arroyo J, Pugachev KV, Miller C, Zhang ZX, Weltzin R et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J Virol 2001; 75: 7290–7304.
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 2012; 380: 1559–1567.
Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372: 113–123.
Deauvieau F, Sanchez V, Balas C, Kennel A, De Montfort A, Lang J et al. Innate immune responses in human dendritic cells upon infection by chimeric yellow-fever dengue vaccine serotypes 1–4. Am J Trop Med Hyg 2007; 76: 144–154.
Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008; 26: 5712–5721.
Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P et al. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc Natl Acad Sci USA 2010; 107: 16922–16927.
Hatch S, Endy TP, Thomas S, Mathew A, Potts J, Pazoles P et al. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J Infect Dis 2011; 203: 1282–1291.
Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE, Shresta S . Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog 2013; 9: e1003723.
Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 2013; 87: 2693–2706.
Capeding RZ, Luna IA, Bomasang E, Lupisan S, Lang J, Forrat R et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine 2011; 29: 3863–3872.
Dayan GH, Garbes P, Noriega F, Izoton De Sadovsky AD, Rodrigues PM, Giuberti C et al. Immunogenicity and safety of a recombinant tetravalent dengue vaccine in children and adolescents ages 9–16 years in Brazil. Am J Trop Med Hyg 2013; 89: 1058–1065.
Lanata CF, Andrade T, Gil AI, Terrones C, Valladolid O, Zambrano B et al. Immunogenicity and safety of tetravalent dengue vaccine in 2–11 year-olds previously vaccinated against yellow fever: randomized, controlled, phase II study in Piura, Peru. Vaccine 2012; 30: 5935–5941.
Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH . Live-attenuated tetravalent dengue vaccine in dengue-naive children, adolescents, and adults in Mexico city: randomized controlled phase 1 trial of safety and immunogenicity. Pediatr Infect Dis J 2011; 30: e9–e17.
Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2–45 y: phase II randomized controlled trial in Singapore. Hum Vaccin Immunother 2012; 8: 1259–1271.
Schlesinger JJ, Brandriss MW, Walsh EE . Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol 1987; 68 (Pt3): 853–857.
Falgout B, Bray M, Schlesinger JJ, Lai CJ . Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol 1990; 64: 4356–4363.
Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K et al. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 2006; 193: 1078–1088.
Guirakhoo F, Kitchener S, Morrison D, Forrat R, Mccarthy K, Nichols R et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVaxTM-DEN2) vaccine: phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vaccin 2006; 2: 60–67.
Barrett AD . Yellow fever vaccines. Biologicals 1997; 25: 17–25.
Jennings AD, Whitby JE, Minor PD, Barrett ADT . Comparison of nucleotide and deduced amino acid sequences of the envelope protein genes of the wild-type French viscerotropic strain of yellow fever virus and the live vaccine strain, French neurotropic vaccine, derived from it. Virology 1992; 192: 692–695.
Martin M, Tsai TF, Cropp B, Chang GJ, Holmes DA, Tseng J et al. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet 2001; 358: 98–104.
Monath TP . Yellow fever vaccine. Expert Rev Vaccines 2005; 4: 553–574.
Acknowledgements
The work was supported in part by a program project grant from the Shanghai Pasteur Health Research Foundation (Y359P41505) to XJ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, H., Lee, M. & Jin, X. Guiding dengue vaccine development using knowledge gained from the success of the yellow fever vaccine. Cell Mol Immunol 13, 36–46 (2016). https://doi.org/10.1038/cmi.2015.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2015.76
Keywords
This article is cited by
-
Semi-field evaluation of the exposure-free mosquito electrocuting trap and BG-Sentinel trap as an alternative to the human landing catch for measuring the efficacy of transfluthrin emanators against Aedes aegypti
Parasites & Vectors (2021)
-
Non-coding RNA: a key regulator of the pathogenicity and immunity of Flaviviridae viruses infection
Cellular & Molecular Immunology (2018)
-
Preventive and therapeutic challenges in combating Zika virus infection: are we getting any closer?
Journal of NeuroVirology (2017)
-
Vaccinations for Neuroinfectious Disease: A Global Health Priority
Neurotherapeutics (2016)