Abstract
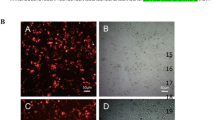
RNA interference (RNAi)-based gene silencing is widely used in laboratories for gene function studies and also holds a great promise for developing treatments for diseases. However, in vivo delivery of RNAi therapy remains a key issue. Lentiviral vectors have been employed for stable gene transfer and gene therapy and therefore are expected to deliver a stable and durable RNAi therapy. But this does not seem to be true in some disease models. Here, we showed that lentivirus delivered short-hairpin RNA (shRNA) against human papillomavirus (HPV) E6/E7 oncogenes were effective for only 2 weeks in a cervical cancer model. However, using this vector to carry two copies of the same shRNA or two shRNAs targeting at two different but closely related genes (HPV E6 and vascular endothelial growth factor) was more effective at silencing the gene targets and inhibiting cell or even tumor growth than their single shRNA counterparts. The cancer cells treated with dual shRNA were also more sensitive to chemotherapeutic drugs than single shRNA-treated cells. These results suggest that a multi-shRNA strategy may be a more attractive approach for developing an RNAi therapy for this cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- RNAi:
-
RNA interference
- shRNA:
-
short hairpin RNA
- HPV:
-
human papillomavirus
- siRNA:
-
short interfering RNA
- VEGF:
-
vascular endothelial growth factor
- LV:
-
lentiviral vector
References
Bitko V, Musiyenko A, Shulyayeva O, Barik S . Inhibition of respiratory viruses by nasally administered siRNA. Nat Med 2005; 11: 50–55.
Dasgupta R, Perrimon N . Using RNAi to catch Drosophila genes in a web of interactions: insights into cancer research. Oncogene 2004; 23: 8359–8365.
Hannon GJ, Rossi JJ . Unlocking the potential of the human genome with RNA interference. Nature 2004; 431: 371–378.
Capodici J, Kariko K, Weissman D . Inhibition of HIV-1 infection by small interfering RNA-mediated RNA interference. J Immunol 2002; 169: 5196–5201.
Jacque JM, Triques K, Stevenson M . Modulation of HIV-1 replication by RNA interference. Nature 2002; 418: 435–438.
Novobrantseva TI, Akinc A, Borodovsky A, de Fougerolles A . Delivering silence: advancements in developing siRNA therapeutics. Curr Opin Drug Discov Devel 2008; 11: 217–224.
de Fougerolles AR . Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther 2008; 19: 125–132.
Goodwin EC, DiMaio D . Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci USA 2000; 97: 12513–12518.
Munger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM . Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. Embo J 1989; 8: 4099–4105.
von Knebel Doeberitz M, Oltersdorf T, Schwarz E, Gissmann L . Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res 1988; 48: 3780–3786.
Jiang M, Milner J . Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene 2002; 21: 6041–6048.
Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F . siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 2003; 22: 5938–5945.
Putral LN, Bywater MJ, Gu W, Saunders NA, Gabrielli BG, Leggatt GR et al. RNA interference against human papillomavirus oncogenes in cervical cancer cells results in increased sensitivity to cisplatin. Mol Pharmacol 2005; 68: 1311–1319.
Gu W, Putral L, Hengst K, Minto K, Saunders NA, Leggatt G et al. Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther 2006; 13: 1023–1032.
Yoshinouchi M, Yamada T, Kizaki M, Fen J, Koseki T, Ikeda Y et al. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol Ther 2003; 8: 762–768.
Fujii T, Saito M, Iwasaki E, Ochiya T, Takei Y, Hayashi S et al. Intratumor injection of small interfering RNA-targeting human papillomavirus 18 E6 and E7 successfully inhibits the growth of cervical cancer. Int J Oncol 2006; 29: 541–548.
Jonson AL, Rogers LM, Ramakrishnan S, Downs Jr LS . Gene silencing with siRNA targeting E6/E7 as a therapeutic intervention in a mouse model of cervical cancer. Gynecol Oncol 2008; 111: 356–364.
Fish RJ, Kruithof EK . Short-term cytotoxic effects and long-term instability of RNAi delivered using lentiviral vectors. BMC Mol Biol 2004; 5: 9.
An DS, Qin FX, Auyeung VC, Mao SH, Kung SK, Baltimore D et al. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther 2006; 14: 494–504.
Tang S, Tao M, McCoy Jr JP, Zheng ZM . Short-term induction and long-term suppression of HPV16 oncogene silencing by RNA interference in cervical cancer cells. Oncogene 2006; 25: 2094–2104.
Zheng ZM, Tang S, Tao M . Development of resistance to RNAi in mammalian cells. Ann N Y Acad Sci 2005; 1058: 105–118.
Gu W, Putral LN, Irving A, McMillan NA . The development and future of oligonucleotide-based therapies for cervical cancer. Curr Opin Mol Ther 2007; 9: 126–131.
Chen J, Irving A, McMillan N, Gu W . Future of RNAi-based therapies for human papillomavirus-associated cervical cancer. Future Virology 2007; 2: 9.
ter Brake O, Konstantinova P, Ceylan M, Berkhout B . Silencing of HIV-1 with RNA interference: a multiple shRNA approach. Mol Ther 2006; 14: 883–892.
Anderson J, Akkina R . CXCR4 and CCR5 shRNA transgenic CD34+ cell derived macrophages are functionally normal and resist HIV-1 infection. Retrovirology 2005; 2: 53.
Brake OT, Hooft K, Liu YP, Centlivre M, Jasmijn von Eije K, Berkhout B . Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol Ther 2008; 16: 557–564.
Henry SD, van der Wegen P, Metselaar HJ, Tilanus HW, Scholte BJ, van der Laan LJ . Simultaneous targeting of HCV replication and viral binding with a single lentiviral vector containing multiple RNA interference expression cassettes. Mol Ther 2006; 14: 485–493.
Wu KL, Zhang X, Zhang J, Yang Y, Mu YX, Liu M et al. Inhibition of Hepatitis B virus gene expression by single and dual small interfering RNA treatment. Virus Res 2005; 112: 100–107.
Yoo JY, Kim JH, Kwon YG, Kim EC, Kim NK, Choi HJ et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther 2007; 15: 295–302.
Sambrook R . Molecular Cloning. 3rd edn., vol. 2. Cold Spring Harbor Laboratory Press: Cold Spring Harbor New York, 1989.
Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM . Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Zhao KN, Gu W, Fang NX, Saunders NA, Frazer IH . Gene codon composition determines differentiation-dependent expression of a viral capsid gene in keratinocytes in vitro and in vivo. Mol Cell Biol 2005; 25: 8643–8655.
Klein D, Bugl B, Gunzburg WH, Salmons B . Accurate estimation of transduction efficiency necessitates a multiplex real-time PCR. Gene Therapy 2000; 7: 458–463.
Gu W, Cochrane M, Leggatt GR, Payne E, Choyce A, Zhou F et al. Both treated and untreated tumors are eliminated by short hairpin RNA-based induction of target-specific immune responses. Proc Natl Acad Sci USA 2009; 106: 8314–8319.
Tang X, Zhang Q, Nishitani J, Brown J, Shi S, Le AD . Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin Cancer Res 2007; 13: 2568–2576.
Le Buanec H, D'Anna R, Lachgar A, Zagury JF, Bernard J, Ittele D et al. HPV-16 E7 but not E6 oncogenic protein triggers both cellular immunosuppression and angiogenic processes. Biomed Pharmacother 1999; 53: 424–431.
Toussaint-Smith E, Donner DB, Roman A . Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene 2004; 23: 2988–2995.
Emanuele S, Lauricella M, Tesoriere G . Histone deacetylase inhibitors: apoptotic effects and clinical implications (Review). Int J Oncol 2008; 33: 637–646.
Kuo PH, Carlson KR, Christensen I, Girardi M, Heald PW . FDG-PET/CT for the evaluation of response to therapy of cutaneous T-cell lymphoma to vorinostat (suberoylanilide hydroxamic acid, SAHA) in a phase II trial. Mol Imaging Biol 2008; 10: 306–314.
Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA 2006; 103: 17372–17377.
Manilla P, Rebello T, Afable C, Lu X, Slepushkin V, Humeau LM et al. Regulatory considerations for novel gene therapy products: a review of the process leading to the first clinical lentiviral vector. Hum Gene Ther 2005; 16: 17–25.
Wang S, Liu H, Ren L, Pan Y, Zhang Y . Inhibiting colorectal carcinoma growth and metastasis by blocking the expression of VEGF using RNA interference. Neoplasia 2008; 10: 399–407.
Shibata MA, Morimoto J, Shibata E, Otsuki Y . Combination therapy with short interfering RNA vectors against VEGF-C and VEGF-A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther 2008; 15: 776–786.
Chen SM, Wang Y, Xiao BK, Tao ZZ . Effect of blocking VEGF, hTERT and Bcl-xl by multiple shRNA expression vectors on the human laryngeal squamous carcinoma xenograft in nude mice. Cancer Biol Ther 2008; 7: 734–739.
Lopez-Ocejo O, Viloria-Petit A, Bequet-Romero M, Mukhopadhyay D, Rak J, Kerbel RS . Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene 2000; 19: 4611–4620.
ter Brake O, t Hooft K, Liu YP, Centlivre M, von Eije KJ, Berkhout B . Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol Ther 2008; 16: 557–564.
Acknowledgements
We thank Dr Ibtissam Abdul Jabbar for her technical support of cell sorting. Funding sources are NHMRC project grant (NM, GL) and NHMRC Peter Doherty Fellowship (WG) and Early Career Researcher Grant (WG) of the University of Queensland, Brisbane, Australia and the Australian Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gu, W., Payne, E., Sun, S. et al. Inhibition of cervical cancer cell growth in vitro and in vivo with dual shRNAs. Cancer Gene Ther 18, 219–227 (2011). https://doi.org/10.1038/cgt.2010.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.72
Keywords
This article is cited by
-
Inhibition of vascular endothelial growth factor with shRNA in chondrocytes ameliorates osteoarthritis
Journal of Molecular Medicine (2016)