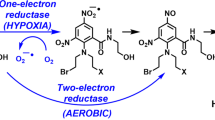
Abstract
Hypoxia is an important factor in tumor growth. It is associated with resistance to conventional anticancer treatments. Gene therapy targeting hypoxic tumor cells therefore has the potential to enhance the efficacy of treatment of solid tumors. Transfection of a panel of tumor cell lines with plasmid constructs containing hypoxia-responsive promoter elements from the genes, vascular endothelial growth factor (VEGF) and erythropoietin, linked to the minimal cytomegalovirus (mCMV) or minimal interleukin-2 (mIL-2) promoters showed optimum hypoxia-inducible luciferase reporter gene expression with five repeats of VEGF hypoxic-response element linked to the mCMV promoter. Adenoviral vectors using this hypoxia-inducible promoter to drive therapeutic transgenes produced hypoxia-specific cell kill of HT1080 and HCT116 cells in the presence of prodrug with both herpes simplex virus thymidine kinase/ganciclovir and nitroreductase (NTR)/CB1954 prodrug-activating systems. Significant cytotoxic effects were also observed in patient-derived human ovarian cancer cells. The NTR/CB1954 system provided more readily controllable transgene expression and so was used for in vivo experiments of human HCT116 xenografts in nude mice. Subjects treated intratumorally with Ad-VEGFmCMV-NTR and intraperitoneal injection of CB1954 demonstrated a statistically significant reduction in tumor growth. Immunohistochemistry of treated xenografts showed a good correlation between transgene expression and hypoxic areas. Further investigation of these hypoxia-inducible adenoviral vectors, alone or in combination with existing modalities of cancer therapy, may aid in the future development of successful Gene-Directed Enzyme Prodrug Therapy systems, which are much needed for targeting solid tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- CMV:
-
cytomegalovirus
- EPO:
-
erythropoietin
- GCV:
-
ganciclovir
- HREs:
-
hypoxic-response elements
- HSVtk:
-
herpes simplex virus thymidine kinase
- IL-2:
-
interleukin-2
- NTR:
-
nitroreductase
- VEGF:
-
vascular endothelial growth factor
References
Brown JM . Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol Med Today 2000; 6: 157–162.
Vaupel P, Mayer A . Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metast Rev 2007; 26: 225–239.
Fechner G, Muller G, Schmidt D, Garbe S, Hauser S, Vaupel P et al. Evaluation of hypoxia-mediated growth factors in a novel bladder cancer animal model. Anticancer Res 2007; 27: 4225–4231.
Binley K, Askham Z, Iqball S, Spearman H, Martin L, de Alwis M et al. Long-term reversal of chronic anemia using a hypoxia-regulated erythropoietin gene therapy. Blood 2002; 100: 2406–2413.
Chadderton N, Cowen RL, Sheppard FC, Robinson S, Greco O, Scott SD et al. Dual responsive promoters to target therapeutic gene expression to radiation-resistant hypoxic tumor cells. Int J Radiat Oncol Biol Phys 2005; 62: 213–222.
Koshikawa N, Takenaga K, Tagawa M, Sakiyama S . Therapeutic efficacy of the suicide gene driven by the promoter of vascular endothelial growth factor gene against hypoxic tumor cells. Cancer Res 2000; 60: 2936–2941.
Kaliberov SA, Buchsbaum DJ, Gillespie GY, Curiel DT, Arafat WO, Carpenter M et al. Adenovirus-mediated transfer of BAX driven by the vascular endothelial growth factor promoter induces apoptosis in lung cancer cells. Mol Ther 2002; 6: 190–198.
Shibata T, Giaccia AJ, Brown JM . Hypoxia-inducible regulation of a prodrug-activating enzyme for tumor-specific gene therapy. Neoplasia 2002; 4: 40–48.
Lee S, Kim K, Kim HA, Kim SW, Lee M . Augmentation of erythropoietin enhancer-mediated hypoxia-inducible gene expression by co-transfection of a plasmid encoding hypoxia-inducible factor 1alpha for ischemic tissue targeting gene therapy. J Drug Target 2008; 16: 43–50.
Okabe S, Arai T, Yamashita H, Sugihara K . Adenovirus-mediated prodrug-enzyme therapy for CEA-producing colorectal cancer cells. J Cancer Res Clin Oncol 2003; 129: 367–373.
Ji X, Zhang J, Cheng L, Wei F, Li H, Liu X et al. Oncolytic adenovirus delivering herpes simplex virus thymidine kinase suicide gene reduces the growth of human retinoblastoma in an in vivo mouse model. Exp Eye Res 2009; 89: 193–199.
Dorer DE, Nettelbeck DM . Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev 2009; 61: 554–571.
Greco O, Dachs GU . Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J Cell Physiol 2001; 187: 22–36.
Wilson WR, Hicks KO, Pullen SM, Ferry DM, Helsby NA, Patterson AV . Bystander effects of bioreductive drugs: potential for exploiting pathological tumor hypoxia with dinitrobenzamide mustards. Radiat Res 2007; 167: 625–636.
Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther 2007; 15: 834–840.
Ahn M, Lee SJ, Li X, Jimenez JA, Zhang YP, Bae KH et al. Enhanced combined tumor-specific oncolysis and suicide gene therapy for prostate cancer using M6 promoter. Cancer Gene Ther 2009; 16: 73–82.
Patel P, Young JG, Mautner V, Ashdown D, Bonney S, Pineda RG et al. A phase I/II clinical trial in localized prostate cancer of an adenovirus expressing nitroreductase with CB1984. Mol Ther 2009; 17: 1292–1299.
Weedon SJ, Green NK, McNeish IA, Gilligan MG, Mautner V, Wrighton CJ et al. Sensitisation of human carcinoma cells to the prodrug CB1954 by adenovirus vector-mediated expression of E. coli nitroreductase. Int J Cancer 2000; 86: 848–854.
Jaberipour M, Vass SO, Guise CP, Grove JI, Knox RJ, Hu L et al. Testing double mutants of the enzyme nitroreductase for enhanced cell sensitisation to prodrugs: effects of combining beneficial single mutations. Biochem Pharmacol 2010; 79: 102–111.
Ingram N, MacCormac LP, Oxley NT, Burns PA, Hall GD . Role of cell surface molecules and autologous ascitic fluid in determining efficiency of adenoviral transduction of ovarian cancer cells. Cancer Gene Ther 2010; 17: 684–693.
Ahn GO, Brown M . Targeting tumors with hypoxia-activated cytotoxins. Front Biosci 2007; 12: 3483–3501.
Greco O, Marples B, Joiner MC, Scott SD . How to overcome (and exploit) tumor hypoxia for targeted gene therapy. J Cell Physiol 2003; 197: 312–325.
Palmer DH, Young LS, Mautner V . Cancer gene-therapy: clinical trials. Trends Biotechnol 2006; 24: 76–82.
Ingram N, Porter CD . Transcriptional targeting of acute hypoxia in the tumour stroma is a novel and viable strategy for cancer gene therapy. Gene Ther 2005; 12: 1058–1069.
Nishihara E, Nagayama Y, Narimatsu M, Namba H, Watanabe M, Niwa M et al. Treatment of thyroid carcinoma cells with four different suicide gene/prodrug combinations in vitro. Anticancer Res 1998; 18: 1521–1525.
Wilson WR, Pullen SM, Hogg A, Helsby NA, Hicks KO, Denny WA . Quantitation of bystander effects in nitroreductase suicide gene therapy using three-dimensional cell cultures. Cancer Res 2002; 62: 1425–1432.
Heinkelein M, Hoffmann U, Lucke M, Imrich H, Muller JG, Meixensberger J et al. Experimental therapy of allogeneic solid tumors induced in athymic mice with suicide gene-transducing replication-competent foamy virus vectors. Cancer Gene Ther 2005; 12: 947–953.
Aghi M, Hochberg F, Breakefield XO . Prodrug activation enzymes in cancer gene therapy. J Gene Med 2000; 2: 148–164.
Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol 2004; 183: 145–154.
Poulios E, Trougakos IP, Gonos ES . Comparative effects of hypoxia on normal and immortalized human diploid fibroblasts. Anticancer Res 2006; 26: 2165–2168.
Braidwood L, Dunn PD, Hardy S, Evans TR, Brown SM . Antitumor activity of a selectively replication competent herpes simplex virus (HSV) with enzyme prodrug therapy. Anticancer Res 2009; 29: 2159–2166.
White CL, Menghistu T, Twigger KR, Searle PF, Bhide SA, Vile RG et al. Escherichia coli nitroreductase plus CB1954 enhances the effect of radiotherapy in vitro and in vivo. Gene Ther 2008; 15: 424–433.
Benouchan M, Do Nascimento F, Perret GY, Colombo BM . Delivery of the bacterial nitroreductase gene into endothelial cells prolongs the survival of tumour-bearing mice by bystander mechanisms. Int J Oncol 2006; 28: 457–462.
Lukashev AN, Fuerer C, Chen MJ, Searle P, Iggo R . Late expression of nitroreductase in an oncolytic adenovirus sensitizes colon cancer cells to the prodrug CB1954. Hum Gene Ther 2005; 16: 1473–1483.
Chen MJ, Green NK, Reynolds GM, Flavell JR, Mautner V, Kerr DJ et al. Enhanced efficacy of Escherichia coli nitroreductase/CB1954 prodrug activation gene therapy using an E1B-55K-deleted oncolytic adenovirus vector. Gene Ther 2004; 11: 1126–1136.
Post DE, Sandberg EM, Kyle MM, Devi NS, Brat DJ, Xu Z et al. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res 2007; 67: 6872–6881.
Cho WK, Seong YR, Lee YH, Kim MJ, Hwang KS, Yoo J et al. Oncolytic effects of adenovirus mutant capable of replicating in hypoxic and normoxic regions of solid tumor. Mol Ther 2004; 10: 938–949.
Post DE, Van Meir EG . Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther 2001; 8: 1801–1807.
Vassaux G, Hurst HC, Lemoine NR . Insulation of a conditionally expressed transgene in an adenoviral vector. Gene Ther 1999; 6: 1192–1197.
Ring CJ, Harris JD, Hurst HC, Lemoine NR . Suicide gene expression induced in tumour cells transduced with recombinant adenoviral, retroviral and plasmid vectors containing the ERBB2 promoter. Gene Ther 1996; 3: 1094–1103.
Plumb J, Norlin C, Young PC . Exposures and outcomes of children with urticaria seen in a pediatric practice-based research network: a case–control study. Arch Pediatr Adolesc Med 2001; 155: 1017–1021.
Andrews PA, Jones JA . Characterization of binding proteins from ovarian carcinoma and kidney tubule cells that are specific for cisplatin modified DNA. Cancer Commun 1991; 3: 93–102.
Workman P, Twentyman P, Balkwill F, Balmain A, Chaplin D, Double J, Embleton J et al. United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia (Second Edition). Br J Cancer 1998; 77: 1–10.
Shnyder SD, Cooper PA, Pettit GR, Lippert III JW, Bibby MC . Combretastatin A-1 phosphate potentiates the antitumour activity of cisplatin in a murine adenocarcinoma model. Anticancer Res 2003; 23: 1619–1623.
Acknowledgements
This work and all authors were supported by Cancer Research UK. We thank Sarah Perry for all the help and advice with the immunohistochemistry and to Luci MacCormac for the primary human ovarian cultures used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Harvey, T., Hennig, I., Shnyder, S. et al. Adenovirus-mediated hypoxia-targeted gene therapy using HSV thymidine kinase and bacterial nitroreductase prodrug-activating genes in vitro and in vivo. Cancer Gene Ther 18, 773–784 (2011). https://doi.org/10.1038/cgt.2011.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2011.43
Keywords
This article is cited by
-
Suicide gene strategies applied in ovarian cancer studies
Cancer Gene Therapy (2023)
-
Lentivirus-mediated RASSF1A expression suppresses aggressive phenotypes of gastric cancer cells in vitro and in vivo
Gene Therapy (2015)