Abstract
Proper control of apoptotic signaling is important for maintenance of testicular homeostasis after ionizing radiation (IR). Herein, we challenged the hypothesis that ghrelin, a pleiotropic modulator, is potentially involved in IR-induced germ cell injury. Lower body exposure to 2 Gy of IR induced a notable increase of ghrelin expression in the nuclear of differentiating spermatogonia at defined stages, with an impairment in the Leydig cells (LCs)-expressing ghrelin. Unexpectedly, inhibition of the ghrelin pathway by intraperitoneal injection of a specific GHS-R1α antagonist enhanced spermatogonia elimination by apoptosis during the early recovery following IR, and thereafter resulted in impaired male fertility, suggesting that the anti-apoptotic effects of evoked ghrelin, although transient along testicular IR injury, have a profound influence on the post-injury recovery. In addition, inhibition of ghrelin signaling resulted in a significant increase in the intratesticular testosterone (T) level at the end of 21 days after IR, which should stimulate the spermatogenic recovery from surviving spermatogonia to a certain extent during the late stage. We further demonstrated that the upregulation and nuclear trafficking of ghrelin, elaborately regulated by IR-elicited antioxidant system in spermatogonia, may act through a p53-dependent mechanism. The elicitation of ghrelin expression by IR stress, the regulation of ghrelin expression by IR-induced oxidative stress and the interaction between p53 and ghrelin signaling during IR injury were confirmed in cultured spermatogonia. Hence, our results represent the first evidence in support of a radioprotective role of ghrelin in the differentiating spermatogonia. The acutely, delicate regulation of local-produced ghrelin appears to be a fine-tune mechanism modulating the balance between testicular homeostasis and early IR injury.
Similar content being viewed by others
Main
Spermatogenesis, a finely tuned process, is very sensitive to endogenous and exogenous stress.1, 2, 3 Cancer therapies such as radiation often lead to a temporary disruption or a complete arrest of spermatogenesis.4 In major cases, detrimental effects of ionizing radiation (IR) on biological tissue are mediated via indirect interactions, which increased production of hydroxyl radicals (•OH) by radiolysis of H2O, followed by the abnormal elevation of cytotoxic reactive oxygen species (ROS) and oxidative damage.5, 6 The latter results in a cell cycle block, followed by apoptosis if the damage is severe. The apoptotic death of selected germ cells (GCs) after irradiation helps to maintain the proper ratio of the GCs to sertoli cells (SCs).7 Although p53 is necessary for radiation-induced GC death, the relevant biochemical events following p53 activation in testis have not been well characterized.8 On the other hand, IR differentially induces apoptosis of GCs, with the actively dividing spermatogonia being the most susceptible.9 To this end, strict maintenance of genomic stability and prevention of excessive damage in spermatogonia are essential for successful outcome of meiosis to assure the fidelity sufficient for proper post-irradiation heredity.
Ghrelin was originally identified as the endogenous ligand of the growth hormone (GH) secretagogue receptor (GHS-R). Since then, an increasing body of evidence demonstrates its potential involvement in male reproduction.10 Ghrelin and its functional receptor are expressed in rat and human testes.11 Ghrelin has been mainly observed in interstitial Leydig cells (LCs).12 Functionally, ghrelin can inhibit the human CG- and cAMP-stimulated testosterone (T) secretion in vitro.13 In addition, we have shown that deregulated ghrelin expression contributes to the pathogenesis of leptin-deficiency induced male infertility.14 Collectively, ghrelin may serve as a pleiotropic modulator of male reproduction.15
Recent works point to a direct participation of ghrelin in the GC apoptosis. For example, ghrelin administration significantly suppressed proliferation-associated peptide PCNA in the spermatogonia as well as spermatocytes.16 Inhibition of ghrelin signaling reduces GC apoptosis and thereby resulted in improved sperm production in ob/ob mice.14 In addition, ghrelin can inhibit expression of the gene encoding stem cell factor (Scf), which acts as the major paracrine survival factor during GC injury.17 Nevertheless, the role and underlying mechanism of local ghrelin in the testicular GCs apoptosis under certain pathological conditions remains elusive. Therefore, the present work attempted to explore the possible involvement of testicular ghrelin in the IR-induced testis injury.
Results
Expression of testicular ghrelin correlates to the increased apoptosis after IR injury
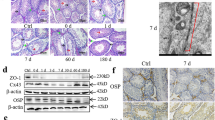
Testicular expression of ghrelin was first explored at the mRNA and protein levels. Initial quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analyses demonstrated a clear-cut elevation in ghrelin level in response to testicular IR treatment, with no difference being detected between two post-treatment time points (Figure 1a). This expression was confirmed at the translational level (Figure 1b). In control mice, ghrelin immunoreactivity was mainly detected in the cytoplasm of LCs, which was substantially abolished by IR. Conversely, ghrelin immunoreactivity was predominantly localized in the nuclear in the outer layer of the seminiferous tubules after IR (Figure 1c). IR-induced ghrelin expression appeared to be restricted to spermatogonia as shown in higher magnification (inset in Figure 1c). In particular, ghrelin first appeared at Stage II–IV, maintained a stable level at Stage V–VI, and reached the highest level at Stage VII–VIII (Supplementary Figure S1). The subcellular localization of IR-induced ghrelin was further confirmed by immunoblotting analysis with cytoplasmic marker Gapdh and nuclear marker histone H1 in the extracts from isolated spermatogonia (Figure 1d and Supplementary Figure S2). Because the killing of male GCs by radiation has previously been attributed to apoptosis7 (we also confirmed that increased apoptosis not aberrant cell proliferation contributes to the cell loss in irradiated testis (Supplementary Figures S3 a–c)) and the apoptosis of the murine GCs after testicular IR reaches to the maximum level at about 16 h (as assessed by the apoptotic index, Figure 1e), we next determined whether the GCs expressing nuclear ghrelin were experiencing apoptosis using double immunofluorescence analysis at this time point. TUNEL-positive cells (empty arrows in Figure 1f), which have been confirmed to be spermatogonia (Supplementary Figure S3d), appeared in the outer layer of the seminiferous tubules at post-treatment 16 h. In contrast, no apoptotic GCs were found positive for ghrelin immunostaining (arrows in Figure 1f). These results are collectively indicative of a potential involvement of ghrelin in IR-induced testicular injury.
IR treatment at a dose of 2 Gy evoked a significant increase in the expression of testicular ghrelin. (a) The relative expression levels of testicular ghrelin at different time points after IR treatment were monitored using quantitative RT-PCR (qRT-PCR) (n=3). (b) Western blotting analysis of ghrelin protein levels at two time points after IR treatment. Actin served as a loading control. (c) Testicular sections from Bouin’s-fixed paraffin-embedded testes were immunostained for ghrelin using a rabbit anti-ghrelin polyclonal antibody. Immunoreactive ghrelin appeared as reddish-brown precipitates and was mainly detected in the Leydig cells (LCs) of control testes and in the spermatogonia (Spg) of irradiated testes, respectively. Sections immunostained with a preabsorbed serum (NC, negative control) showed no positive signals, demonstrating the specificity of this antibody. Higher magnifications of the basal spermatogonia layer of the tubules are shown as insets. Spc, spermatocyte; Esd, elongating/elongated spermatid. Bar=20 μm. (d) After exposure, Spg were subjected to cytoplasmic and nuclear fractionation analysis followed by immunoblotting analyses as described in Materials and Methods. (e) The apoptotic index was determined by the percentages of seminiferous tubule with more than three TUNEL-positive cells. More than 200 tubules were scored for TUNEL positivity. Data were presented as mean±S.E.M. from at least three independent experiments. (f) Combined TUNEL (empty arrows) and double immunofluorescent labeling indicates non-apoptotic germ cells expressing ghrelin (arrows) at the end of 16 h after IR treatment. Nuclear are demonstrated using DAPI staining. Bar=20 μm
Inhibition of ghrelin signaling enhances the IR-induced testicular mitotic damage
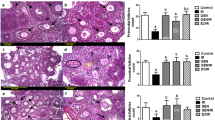
Subsequent analyses were directed toward the characterization of the role of endogenous ghrelin signaling in IR-induced GC injury. We treated mice with [D-Lys-3]-GHRP-6 (D-GHRP) (Figure 2a), a specific GHS-R1α antagonist, which had been shown to effectively inhibit the effects of ghrelin within the body.14 We first demonstrated that D-GHRP treatment alone exerted no apparent toxicity to the GCs development (Supplementary Figure S4). After D-GHRP treatment, very few GCs inside the seminiferous tubules were found positive for the nuclear staining at post-treatment 16 h, suggesting that disruption of the interaction between ghrelin and its receptor can efficiently block the ghrelin translocation in response to IR insult (Figure 2b). Subsequent quantitative TUNEL analyses revealed that inhibition of ghrelin signaling significantly enhances the IR-induced GCs apoptosis at post-treatment 16 h, with no difference being detected at post-treatment 21 days (Figure 2c). As shown in Figures 2d–f, the relative percentages of primary spermatocytes (4C) in vehicle and D-GHRP treated groups at Post-IR 16 h were both significantly increased as compared with that in control mice treated with D-GHRP alone (P<0.05). In contrast, only a notable increase was observed in the relative percentages of spermatogonia (2C) in D-GHRP-treated groups at post-IR 16 h. As for post-IR 21 days, the relative percentages of 4C and spermatids (1C) were both significantly reduced in vehicle+IR or D-GHRP+IR-treated groups, with a significantly lower value of 1C being detected in D-GHRP+IR groups. The effect of D-GHRP treatment on IR-induced GCs loss was also morphologically confirmed in hematoxylin and eosin staining (Supplementary Figure S5). These results indicated that the genetic damage induced by IR probably originates from spermatogonia, and may not be effectively repaired in the absence of ghrelin signaling and thereafter manifest itself in late stage of spermatogenesis. To further identify at which stage the differentiation of spermatogonia was blocked, we performed qRT-PCR analyses on testis RNA from D-GHRP or vehicle control-pretreated mice at post-IR 16 h using specific primers for mouse Zbtb16 and Pou5f1 (undifferentiated spermatogonia), for mouse Kit and Sohlh2 (differentiating spermatogonia) and for mouse Ldh-c4 (preleptotene spermatocytes), respectively.18, 19, 20 All cell markers tested proved to be constantly expressed at post-IR 16 h, with the significant decreases only being detectable in the differentiating spermatogonia of D-GHRP-pretreated groups (Figure 2g). These data suggest that the protective effect of endogenous ghrelin against testicular IR insult mainly occurred in the differentiating spermatogonia. The available data made us wonder whether the surviving spermatozoa are capable of inseminating an egg, thereby retaining their reproductive competence. We carried out the mating experiments at the end of 45 days after IR treatment because spermatogenesis is considered to be completely reconstituted around this time point after a low-dose irradiation.21 Overall, the fertility potential was substantially restored in the vehicle control-pretreated mice. By contrast, inhibition of ghrelin signaling resulted in a dramatic decrease in the impregnation rate (Figure 2h). These results convincingly confirmed that activation of ghrelin signaling in spermatogonia is an indispensable defensive mechanism against testicular IR insult.
The transient anti-apoptotic effect of endogenous ghrelin on differentiating spermatogonia is required for their early recovery from testicular IR stress (2 Gy). (a) Schematic representation of the experimental procedure used in the current study. (b) Abolishment of IR-induced ghrelin nuclear translocation by intraperitoneal injection of a specific GHS-R1α antagonist was observed at the end of 16 h after IR treatment by immunohistochemical staining. LC, Leydig cell; Spg, spermatogonia. Bar=20 μm. (c) Quantification of the apoptotic index in ctrl vehicle or D-GHRP-pretreated groups, as described above, at different time points after IR stress. Data were presented as mean±S.E.M. from at least three independent experiments. (d–f) Relative change of germ cell percentage 16 h/21 days after IR was examined by flow cytometry. (g) The deleterious effects of ghrelin inhibition on the spermatogenic differentiation at post-IR 16 h was evaluated by qRT-PCR analyses using specific primers for Zbtb16 and Pou5f1 (undifferentiated spermatogonia), for Kit and Sohlh2 (differentiating spermatogonia) and for Ldh-c4 (preleptotene spermatocytes), respectively. Gapdh served as an internal control. Results presented as mean±S.E.M. of three independent experiments. *P<0.05. (h) Fertility tests in ctrl vehicle or D-GHRP-pretreated mice were carried out as described in Materials and Methods
Effects of ghrelin inhibition on spermatogenesis-related hormones after IR injury
Levels of hormones including T, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were assessed given their pivotal roles in the control of spermatogenesis.22 Despite the reported inhibitory effect of the ghrelin signaling on testicular steroidogenesis,13 mice maintained LH, T and FSH concentration fairly constant along the first 16 h after irradiation (Figures 3a–d). By contrast, the level of intratesticular T remained significantly high at post-IR 21 days in both groups, with the highest level being detected in the D-GHRP-treated groups at this time point (Figure 3a). Consistently, a significant decrease of the plasma LH could also be detected in both groups at post-treatment 21 days, with the lower value observed in the D-GHRP-treated groups (Figure 3c). Moreover, the plasma FSH levels in two experimental groups were both substantially elevated at post-IR 21 days, with no difference being detectable between different groups (Figure 3d). These data suggest that ghrelin signaling may be involved in the serological change during the late recovery from testicular IR injury.
Effects of suppression of ghrelin pathway on spermatogenesis-related hormones levels after testicular IR injury (2 Gy). At different time points after IR treatment, serum and testicular extracts were obtained from different experimental groups as indicated, and intratesticular testosterone (T) (a), and plasma T (b), LH (c), FSH (d) were assayed as described in the Materials and Methods. Results are mean±S.E.M. of five animals per group. A probability of P<0.05 was considered statistically significant
Regulation of ghrelin expression by testicular oxidative stress in response to IR injury
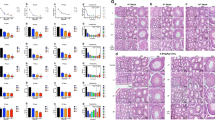
Testicular IR induced oxidative stress, which was characterized by the elevated generation of malondialdehyde (MDA) (Figure 4a) and increased expression of antioxidants (Figures 4b and c).23 The supplementation of exogenous antioxidants (Vit C+Vit E) could significantly reduce IR-induced testicular oxidative stress (Figures 4a–c). Interestingly, IR evoked a notable rise in ghrelin expression at post-IR 16 h, whereas repeated administration of Vit C+Vit E prior to irradiation significantly compromised this upregulation (Figure 4d). Consistently, only a small fraction of nuclear-positive staining was observed in Vit C+Vit E-pretreated mice at post-IR 16 h (Figure 4e). To determine whether oxidant system or antioxidant system regulates ghrelin expression during IR injury, we examined the ghrelin expression following low-dose irradiation (2 Gy) or high-dose irradiation (5 Gy). As increased intension of irradiation, the significant increased MDA concentration (Supplementary Figure S6a), downregulation of glutathione peroxidase (GPX) (Supplementary Figure S6b) and total superoxide dismutase (T-SOD) level (Supplementary Figure S6c) at post-IR 16 h implied severe damage in the testes. High-dose irradiation dramatically reduced the IR-elicited ghrelin expression in testes (Supplementary Figure S6d). Consistently, the nuclear staining ghrelin frequently observed at specific stages following low-dose irradiation was barely detectable after high-dose irradiation (Supplementary Figure S6e). To further define the specific antioxidant signaling, which may regulate ghrelin expression during IR, we treated mice with T-SOD inhibitor 10 min before irradiation. Surprisingly, inhibition of T-SOD activity substantially abolished the IR-induced upregulation (Figure 4f) and nuclear translocation (Figure 4g) of testicular ghrelin at post-IR 16 h. The modulation of oxidative signaling on IR-induced ghrelin expression was further confirmed using immunoblotting analyses in spermatogonia nuclear extracts (Figure 4h). Thus, T-SOD activation elicited by oxidative stress may act on the top of ghrelin signaling during the early recovery after IR stress.
Testicular oxidative stress regulates ghrelin expression during IR injury (2 Gy). Effects of IR treatment along with antioxidants supplement on oxidative stress parameters including malondialdehyde (MDA) (a), glutathione peroxidase (GPX) (b) and total superoxide dismutase (T-SOD) (c) were determined 16 h after irradiation as described in the Materials and Methods. Data were presented as mean±S.E.M. from at least three independent experiments. (d) Effects of antioxidants supplement on IR-elicited ghrelin expression level were measured using western blotting at post-IR 16 h. Actin served as a loading control. (e) Effects of antioxidants supplement on IR-elicited ghrelin expression pattern were evaluated using immunohistochemistry. Spg, spermatogonia. Bar=20 μm. (f) Effects of diethyldithiocarbamic acid sodium salt trihydrate (DEDT) treatment on IR-induced ghrelin expression level were measured using western blotting at post-IR 16 h. (g) Effects of DEDT treatment on IR-induced ghrelin expression pattern were evaluated using immunohistochemistry. Bar=20 μm. (h) Effects of DEDT, Vit C+E or D-GHRP treatment on IR-induced ghrelin expression pattern were verified in the nuclear extracts from cultured spermatogonia (Spg) using western blotting at post-IR 16 h. Lower panels represent the blots reprobed for the nuclear marker, namely hisone H1
Requirement of p53 transactivation in IR-induced ghrelin expression
P53-dependent apoptosis accounted for the bulk of the loss of the differentiating spermatogonia after IR.24 In this context, we examined the association between endogenous ghrelin and p53 in testicular nuclear extracts at post-IR 16 h. The interaction could be detectable only in the presence of IR stress (Figure 5a). Similarly, in cultured spermatogonia, IR stress elicited a notable interaction between p53 and ghrelin, which could be substantially compromised by the treatment with Vit C+ Vit E, DEDT or D-GHRP, respectively (Figure 5b). We then treated mice with a specific chemical inhibitor of p53 pathway prior to the IR treatment. As expected, a single intraperitoneally (i.p.) injection of PFTα 5 days prior to the IR experiments impaired activation of endogenous cellular p53-responsive genes, including cyclin G, p21/waf1/cip1 and mdm2 (Supplementary Figure S7a), and consequently reduced the GC apoptosis at post-IR 16 h (Supplementary Figure S7b). Consistently, few spermatogonia in the PFTα-treated testes were found positive for ghrelin staining (Figure 5c). The inhibitory effect of PFTα on ghrelin nuclear translocation upon IR stress was confirmed using immunoblotting analyses in the extracts from isolated spermatogonia (Figure 5d). Moreover, inhibition of ghrelin signaling appeared to have no significant effect on IR-induced p53 pathway, as determined by qRT-PCR analyses using two p53-responsive genes (Figure 5e). These results suggest that the translocation of testicular ghrelin may achieve via a p53-dependent mechanism.
Requirement of p53 transactivation in IR treatment (2 Gy)-induced nuclear translocation of testicular ghrelin. (a) Enhancement of the association between testicular ghrelin and endogenous p53 in response to IR treatment was demonstrated using a Co-immunoprecipitation (Co-IP) assay followed by western blotting analysis at 16 h after irradiation. Testicular lysates (30 μg of protein, no Co-IP) and Co-IP performed with normal goat (Gt) IgG served as positive and negative controls, respectively. (b) Effects of Vit C+E, DEDT or D-GHRP treatment on IR-induced interaction between ghrelin and p53 were verified in the nuclear extracts from cultured spermatogonia (Spg) using Co-IP followed by immunoblotting analyses at post-IR 16 h. (c) Effects of PFTα pretreatment on IR-induced ghrelin expression pattern were determined using immunohistochemistry at 16 h after IR. LC, Leydig cell; Spg, spermatogonia. Bar=20 μm. (d) Effects of PFTα pretreatment on IR-induced ghrelin nuclear translocation were verified in the nuclear extracts from cultured spermatogonia (Spg) using western blotting at post-IR 16 h. Lower panels represent the blots reprobed for the nuclear marker, namely hisone H1. (e) Effects of ghrelin inhibition on p53 activity upon IR stress were evaluated using qRT-PCR analyses with two p53 pathway molecules in D-GHRP or vehicle ctrl-treated testes. Groups with different superscript letters are statistically different (P<0.05)
Discussion
In the present study, a single IR induced a dramatic increase in the ghrelin expression level at both time-points studied. However, upregulation of ghrelin expression was only positively correlated to the elevated apoptosis at post-IR 16 h (Figure 1e), suggesting that the effect of ghrelin upregulation on GC injury mainly occurs during the very early phase after IR stress. In favor of this assumption, previous studies have demonstrated the involvement of exogenous ghrelin in the acute injury in other tissues. Intravenous injection of ghrelin could attenuate sepsis-induced acute lung injury and mortality.25 Similarly, ghrelin exerts a hepatoprotective effect on carbon tetrachloride-induced acute liver injury.26 In this context, it is possible that enhanced production of endogenous ghrelin in irradiated testis may serve as a rapid response mechanism.
One distinguishing feature in our study is a dramatic decrease of ghrelin expression in LCs took place immediately after IR (Figure 1c). This is understandable as the pathological conditions of irradiated testes are usually counter-conducive to LCs status.27 However, impairment of ghrelin expression in LCs apparently did not cause much deleterious effects on LCs function during the early recovery, because a significant increase in the intratesticular T level can only be detectable at post-IR 21 days, with a higher value being observed in the D-GHRP-pretreated groups (Figure 3a). This observation is in line with the previous report that LCs are more resistant to irradiation injury when compared to GCs.28 The relevance of this observation is two-fold: (1) it further documents the ability of ghrelin to negatively modulate testicular steroidogenesis; (2) it demonstrates for the first time that testis may use the upregulation of intratesticular T level, which may result from the downregulation of ghrelin expression, as a self-recovery promoting mechanism, as it has been shown that continuous treatment with T after IR markedly stimulated the recovery of spermatogenesis.29
IR efficiently induces death of GCs, with the actively dividing spermatogonia being the most susceptible; doses as low as 0.1 Gy are known to cause damage to these cells.9 In our experiments, ghrelin expression in spermatogonia was dramatically increased after IR treatment (Figures 1c and d), suggesting a direct involvement of this molecule in local control of premeiotic homeostasis upon IR insult. Our localization result was not in agreement with the previously reported presence of ghrelin in rodent testis, in which ghrelin was found to express dominantly in LCs.12 Three reasons may help to explain this expression discrepancy. (i) Expression of GHS-R1α has been demonstrated in the tubular compartment of the testis.14 So theoretically, ghrelin may directly participate in the local regulation of GC function. (ii) Redistribution of proteins within the various cell compartments presents an important way of regulating the cellular responsiveness to stress conditions.30 In this sense, trafficking of ghrelin following irradiation emphasizes its unique action as an intrinsic autocrine/paracrine modulator during IR injury. (iii) IR-induced specific ghrelin signals were exclusively observed in spermatogonia at defined stages of the seminiferous epithelial cycle (at stages II–VIII). The physiological meaning of such a staged expression pattern awaits further investigation, although it is tempting to point out that these stages correlated well to the development of spermatogonia.31 Thus, ghrelin may exert certain effects on testicular mitosis in response to IR stress.
Further evidence for a functional role of elevated ghrelin expression is provided by its ability to elicit protective effect on differentiating spermatogonia in the presence of testicular IR. Inhibition of the interaction between ghrelin and its receptor using D-GHRP efficiently suppressed the nuclear translocation of ghrelin from post-irradiation 16 h onwards (Figure 2b) but only selectively resulted in an upregulated apoptosis at post-irradiation 16 h (Figure 2c), suggesting that the transient protective effect of ghrelin on GCs mainly occurs during the early recovery phase. Subsequent FACS analyses further confirmed that spermatogonia were the cell types most vulnerable to radiation-induced damage (Figures 2d–f), which points to a direct cause-and-effect relationship between the disruption of ghrelin signaling and IR-induced premeiotic damage. Specificity of the protective effects of ghrelin upon testicular IR stress is indirectly shown by the fact that inhibition of ghrelin signaling significantly enhanced the impairment of the expressions of two markers for differentiating spermatogonia, namely Kit and Sohlh2 (Figure 2g), indicating that the differentiating spermatogonia are probably the main action sites of ghrelin during IR injury. Of note, at post-IR 45 days, when spermatogenesis is supposed to be fully reconstituted, the impregnation rate of D-GHRP-treated mice was still much lower than that of vehicle-treated mice (Figure 2h). Thus, the protective effects of ghrelin, though transient along testicular IR injury, have a profound influence on the post-injury recovery of male fertility.
Testicular expression of ghrelin in response to IR appeared to be exquisitely regulated. First, expression levels of ghrelin were significantly downregulated immediately after antioxidant supplementation (Vit C+Vit E; Figures 4d, e and h), which suggests that IR-induced ghrelin expression is tonically modulated (either positively or negatively) by oxidative stress. Indeed, deregulated ghrelin levels have been frequently associated with oxidative stress in different human pathologies.32, 33, 34 Furthermore, the exogenous ghrelin can exert protective effects against oxidative stress under different pathological conditions.35 Our findings extend these understanding by identifying IR-elicited oxidative stress as a potent stimulus of testicular ghrelin expression. However, antioxidant system rather than oxidative damage per se appeared to play a more prominent role as the elicitor of testicular ghrelin during IR injury (Supplementary Figure 6). This contention may also explain why IR-induced ghrelin expression only occurs during the very early phase of recovery, as the antioxidant system is usually compromised at a later stage of tubular damage.36 Concerning the enzymatic constituents of this defense system, SOD functions as the first and rate-limiting step of rapid conversion of superoxide anion (O2−.) to hydrogen peroxide (H2O2) in order to prevent highly pernicious hydroxyl radicals.36 Recent studies reveal that SOD plays an important role against testicular oxidative damage in spermatogonia.23 In our study, SOD appeared to be the specific antioxidant signaling modulating ghrelin expression during IR injury (Figures 4f–h). Our results also indicate that the protective effect of SOD system may execute via the regulation of local-produced ghrelin. From a general standpoint, it is intriguing to note that, although testicular oxidative stress is known to be detrimental to spermatogenesis in a number of pathological conditions, ghrelin action in spermatogonia appears to be a specific response to the IR insult (Supplementary Figure 8).
Structurally, we did not find any nuclear localization signal (NLS) in ghrelin amino acid sequence. So, how did IR-elicited ghrelin translocate from the cytoplasm to the nuclear? One possibility is that ghrelin travels with the assistance of other trafficking regulators in the presence of IR stress. This is very common in other apoptosis regulators such as Rap1 and Ndrg2.30, 37 Radiation-induced oxidative stress transiently upregulates p53 expression and induced nuclear translocation of p53 in actively dividing spermatogonia, where p53 functions as a transcription factor that regulates the expression of stress-response genes.38 Consistently, we showed that the nuclear interaction between endogenous ghrelin and p53 could only be detectable in irradiated testes and cultured spermatogonia (Figures 5a and c) and in vivo inhibition of p53 signaling substantially abolished the IR-induced nuclear relocation of ghrelin in spermatogonia (Figure 5c). Regarding the directionality of IR-elicited p53 and ghrelin signaling, we reason that (i) IR-evoked ghrelin expression may require p53 as the functional assistance mechanism, similar as the requirement of p53 by ghrelin signaling in other pathogenesis;39, 40 (ii) on the other hand, ghrelin may operate as a relatively independent signaling during testicular IR injury, because inhibition ghrelin by in vivo D-GHRP treatment had no effects on p53 activity (Figure 5e). Additionally, p53 has been reported to be upregulated by oxidative stress, followed by the p53-dependent transcriptional induction of several target genes involved in the induction of apoptosis.41, 42 It is a logical hypothesis that apoptosis and nuclear translocation of ghrelin following radiation are both p53-dependent events.
In summary, we propose a model in which IR induces DNA damage within the differentiating spermatogonia, and in which p53, an intracellularly activated apoptotic protein, is stabilized by oxidative stress within GCs in response to the injury. This, in turn, leads to the activation of the antioxidant system, followed by a nuclear accumulation of ghrelin. The latter may serve as a mutual antagonist of p53 and help to maintain proper apoptotic balance in differentiating spermatogonia. On the other hand, the impairment of intratesticular environment by IR injury results in the downregulation of ghrelin expression in LCs and the subsequent increase in androgen production during the later phase, which may enhance the recovery of remaining GCs after testicular IR injury. Overall, the delicate regulation of local-produced ghrelin appears to be a fine-tune mechanism modulating the balance between testicular homeostasis and testicular IR injury (Figure 6).
Materials and Methods
Animals and drugs
Wild-type C57BL/6 male mice were obtained from the Animal Research Center of Fourth Military Medical University and maintained on a 12-h light/12-h dark in a 20–25 °C environment, with free access to standard pellet mouse chow and tap water. They were allowed to acclimatize for 10 days before the experiment. In all experiments, mice were killed under diethyl ether anesthesia, followed by cervical dislocation. Testes were immediately removed and decapsulated (free of surrounding epididymal fat). For histological studies, testes were fixed in Bouin’s solution for 24 h and embedded in paraffin. For biochemical analysis, testes were frozen in liquid nitrogen and stored at –80 °C until processing. All animal work was approved by the Animal Care and Use Committee of Fourth Military Medical University and the protocols were strictly conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85–23, revised 1996). All efforts were made to minimize suffering. [D-Lys-3]-GHRP-6 (D-GHRP), a specific GHS-R1α antagonist, was purchased from Sigma-Aldrich (Beijing, China).Vitamin C and vitamin E were obtained from Sigma Diagnostics (St Louis, MO, USA) and Sigma-Aldrich (Beijing, China), respectively. Diethyldithiocarbamic acid sodium salt trihydrate (DEDT), an inhibitor of T-SOD, and pifithrin-α (PFTα), a specific chemical inhibitor of p53 activity, were both provided by Santa Cruz Biotech (Dallas, TX, USA).
Experimental designs
In Experiment 1, effects of IR on ghrelin expression were studied at the transcriptional and translational levels from two time points (n=5). The first time point (16 h) was chosen because apoptosis of the murine testicular GCs, which starts approximately 8 h after IR, increases to maximum effect at about 16 h. The second time point (21 days) was selected because differentiation of mouse testicular GCs from early spermatogonia to spermatocytes and to spermatids lasts approximately 9 and 18 days, respectively. To this end, effects of IR on spermatogenesis could be reflected from the reduction in the 4C population of spermatocytes and the 1C population of spermatids at the 21st day after irradiation.9 In addition, testicular samples were taken at 16 h after IR, and processed for immunohistochemical and double immunofluorescent analyses of ghrelin expression pattern, as described below. In addition, effects of IR treatment on ghrelin nuclear translocation were also verified in cultured spermatogonia.
The involvement of endogenous ghrelin in response to testicular IR stress was evaluated in Experiment 2. To this end, mice were injected i.p. with either D-GHRP (Sigma-Aldrich, 0.1 ml/10 g of bodyweight; n=15) or vehicle control (0.9% sterile saline; n=15) on daily basis for 50 days. At the 5th day after D-GHRP/vehicle control treatment, animals were subjected to IR experiments and testicular tissues were harvested at different time points. Testicular ghrelin expression pattern, studied by immunohistochemistry, was compared to the IR-induced testicular apoptosis. In addition, the effects of disruption of ghrelin signaling on GC composition and mitotic differentiation after IR were determined by flow cytometry and qRT-PCR, respectively, in Experiment 3. We then studied the influence of the ghrelin inhibition on mouse fertility 45 days after IR treatment in Experiment 4, given that murine spermatogenesis has been shown to be completely reconstituted at this time point after low-dose irradiation.21 Meanwhile, serum or intratesticular levels of T, LH and FSH were monitored in D-GHRP/vehicle control-treated mice at different time points after IR in Experiment 5.
The ability of IR-induced oxidative stress to regulate testicular expression of ghrelin was monitored in Experiment 6. To this end, mice were supplemented with vitamin C (4.8 mg/day) and vitamin E (3.6 IU/day) 7 days prior to IR treatment, and mice were maintained on the supplement for additional 2 days after IR treatment. The supplement was prepared fresh daily in liquid form, soaked onto a small piece of bagel, and allowed to dry (dry weight of supplement=130 mg per mouse based on a 35 g mouse). The bagel bits were rapidly eaten, ensuring mice obtained full and equivalent doses. Subsequently, expression profile of testicular ghrelin was explored using immunoblotting and immunohistochemistry. To further characterize the role of antioxidant systems and oxidative stress in the IR-induced ghrelin expression, testicular samples were taken from low dose- (2 Gy) or high dose- (5 Gy) irradiation-treated mice at the end of 16 h after IR, and processed for ghrelin expression analysis using immunoblotting and immunohistochemistry in Experiment 7. Of note, concentrations of MDA and enzymatic antioxidant activities including T-SOD and GPX were monitored spectrophotometrically in both Experiments 6 and 7. Furthermore, the potential involvement of T-SOD in the regulation of IR-induced ghrelin expression was explored in Experiment 8. Mice were injected with DEDT (200 mg/kg, i.p.) 10 min before IR. Expression levels of ghrelin were then assayed at the end of 16 h after IR by means of immunoblotting and immunohistochemistry. In the above-mentioned experiments, paired vehicle-injected animals served as controls.
In Experiment 9, effects of testicular oxidative stress on IR-induced ghrelin expression were confirmed in the cultured spermatogonia. Additionally, in Experiment 10, the requirement of p53 signaling for the IR-induced ghrelin expression was determined in vivo using a specific chemical inhibitor of p53 activity, namely PFTα. Mice received a single i.p. injection of 2.2 mg/kg of PFTα 10 min before irradiation. At the end of 16 h after IR, testis samples were processed for immunohistochemical analysis. In the mean time, the inhibition of PFTα on IR-induced interaction between p53 and ghrelin was confirmed by co-immunoprecipitation (Co-IP) and immunoblotting analyses in both testicular tissues and cultured spermaogonia. Finally, in Experiment 11, the directionality of p53 and ghrelin interaction in response to IR stress was evaluated in D-GHRP-treated testes using qRT-PCR with two p53 pathway molecules.
IR experiments
IR experiments were carried out as described previously.7 Briefly, unanesthetized mice were restrained in polystyrene chambers, and the upper two-thirds of the body were shielded with 3 mm of lead. Animals or cultured spermatogonia received a dose of 2.0 Gy (unless otherwise indicated) irradiation at a rate of 1.04–1.68 Gy/min using a 320-kVp Philips industrial X-ray machine (GMBH, Hamburg, Germany). After exposure, cells were subjected to cytoplasmic and nuclear fractionation analysis using the Subcellular Protein Fractionation Kit for Cultured Cells (Thermo Scientific, Beijing, China) according to the manufacturer’s instructions.
Hormone assays
Mice were killed at different time points as described above. A mid-line sternotomy was performed and 1 ml of blood was drawn by cardiocenthesis. After 15 min of centrifugation at 3000 × g, the serum was collected and stored at −20 °C until analysis. Levels of T, LH and FSH were measured by RIA in plasma or in supernatants of total testicular homogenates as described previously.43 All samples were assayed in duplicate, and each experimental data point consisted of 3–5 samples. Intra- and inter-assay coefficients of variation were approximately 8.3 and 7.8%, respectively, for T; 6.3 and 5.5% for LH; and 4.9 and 2.6% for FSH.
Assessment of male fertility
The reproductive capacities of experimental mice were evaluated by mating one male with two females for a 2-week duration as described previously.43 Female mice were checked for vaginal plugs each morning and litter sizes were recorded on delivery from three successive matings.
Evaluation of oxidant and antioxidant statuses
The oxidant and antioxidant statuses were determined in the tissue homogenates as described elsewhere.23 Briefly, fresh testicular tissues were homogenized in ice-cold NaCl solution. The homogenate was centrifuged at 1700 × g at 4 °C for 15 min. Subsequently, the MDA, GPX and T-SOD levels in the supernatant were assayed spectrophotometrically using commercial kits (Jiancheng Bioengineering, Nanjing, China), and absorbance was read on a standard microplate reader (#680; Bio-Rad Laboratories, Hercules, CA, USA), using 532 nm for MDA, 412 nm for GPX and 560 nm for T-SOD as the primary wavelengths, respectively.
qRT-PCR
Total RNA was extracted from murine testes using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Routine DNase (Applied Biosystems/Ambion, Austin, TX, USA) treatment (1 unit of DNase I/μg of total RNA) was performed before reverse transcription (RT). First-strand cDNA was synthesized with Superscript III (Rnase H-Reverse Transcriptase; Invitrogen, Beijing, China) and PCR was set up according to Promega’s reverse transcription system protocol. The primers used are listed in Supplementary Table 1. The amplification of Gapdh was served as the internal control. PCR products were then quantified by SYBR green intercalation using the MiniOpticon System (Bio-Rad). Gapdh was used to obtain the ΔΔCt values for the calculation of fold increases.
In situ end labeling of fragmented DNA (TUNEL)
Apoptotic cells in testicular sections were identified using In Situ Cell Death Detection Kit, POD (Roche Applied Science, Mannheim, Germany) following the manufacturer’s instructions. Briefly, slides were saturated with 50 μl of an equilibration buffer (EB: 200 mM K-cacodylate, 25 mM Tris-HCl, pH 6.6, 0.2 mM DTT, 0.25 μg/μl bovine serum albumin (BSA), 2.5 mM CoCl2) under a plastic coverslip (Millipore, Beijing, China) to prevent evaporation for 10 min, followed by the incubation with the TUNEL reaction mixture and 1 μl of 25 Units/μl of terminal deoxynucleotidyl transferase. The TUNEL reaction mixture was evenly spread under a plastic coverslip, and slides were incubated at 37 °C for 1 h. The reaction was terminated by immersing the slides in 2 × sodium chloride and sodium citrate (2 × SSC: 300 mM NaCl, 30 mM Na-citrate, pH 7.4) for 15 min at room temperature. Quantification of relative apoptotic rate in testicular sections was carried out as described elsewhere.7 Briefly, slides were blinded, and each tissue section was examined for TUNEL-positive cells, and the apoptotic index was determined by the ratio of the number of essentially round seminiferous tubules with more than three TUNEL-positive cells relative to the total number of essentially round tubules. For testis section, at least 200 tubules were scored for TUNEL positivity. Fluorescent microscopic images were visualized on an inverted microscope (Axio Imager M1 microscope, Zeiss, Göttingen, Germany). Images were downloaded into and finally assembled by Photoshop 7.0 imaging software (Adobe Systems, San Jose, CA, USA).
Flow cytometry
GCs were released from seminiferous tubules in PBS according to the previous report.43 After GCs were stained with 25 mg/l ethidium bromide (Sangon Biotech, Shanghai, China), samples were analyzed by flow cytometer (BD Biosciences, San Jose, CA, USA) with an excitation wavelength of 488 nm.
Co-IP
Testicular tissues were collected at the end of 16 h after IR treatment. Lysates were obtained by homogenizing tissues in IP lysis buffer (10 mM Tris, 0.15 M NaCl, 1% Nonidet P-40, and 10% glycerol, pH 7.4 at 22 °C) supplemented with protease and phosphatase inhibitor mixtures (Roche Diagnostic, Mannheim, Germany), sonicating, and centrifuging at 15 000 × g for 20 min to obtain the clear supernatant. The lysates (∼200 μg) were incubated with goat-anti p53 or control goat (Gt) IgG antibodies at 4 °C overnight. On the following day, protein A-Sepharose (Pierce, Rockford, IL, USA) was added into lystates and the compound was incubated at 4 °C for another 2 h. Immunocomplexes were finally eluted from the sepharose beads by boiling in Laemmli sample buffer and subjected to SDS-PAGE of immunoblotting analysis with rabbit-anti ghrelin antibody as described below. Immunoblotting analysis using testicular lysates (30 μg of protein, no Co-IP) served as positive controls.
Western blotting
Protein samples were prepared in ice-cold RIPA buffer (Tris-HCl 50 mM, NaCl 150 mM, Triton X-100 1%, vol/vol, sodium deoxycholate 1%, wt/vol, and SDS 0.1%, wt/vol, pH 7.5) supplemented with complete proteinase-inhibitor cocktail tablets (Roche Diagnostic, Shanghai, China). Protein estimation was performed by spectrophotometry with a Bio-Rad DC Protein Assay Kit using BSA as a standard and a Bio-Rad Model 680 Plate Reader. A quantity of 30 μg of total protein was separated on 8–15% SDS-PAGE and transferred to nitrocellulose membrane (Millipore). Membranes were then incubated with different primary antibodies, as indicated in Supplementary Table 2, in blocking solution overnight at 4 °C. Final signals were detected using an ECL kit (Amersham Biosciences, Shanghai, China).
Immunohistochemistry
The streptavidin–biotin complex method was employed in the immunohistochemical assay on serial 5-μm sections as previously described.44 Briefly, sections were deparaffinaged, rehydrated to distilled water, and immersed in boiling citrate buffer for 10 min to retrieve antigen. After endogenous peroxidase activity had been blocked with 0.3% H2O2 in methanol for 30 min, slides were incubated with the primary antibody diluted in PBS, at 4 °C overnight in a moist box. Biotinylated IgG was incubated on the sections for 1 h at room temperature and detected with streptavidinperoxidase complex. Peroxidases were detected with 0.7 mg/ml 3-3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA) in 1.6 mg/ml urea hydrogen peroxide. Control slides were incubated with a preabsorbed serum instead of primary antibody.
Combined TUNEL and double immunofluorescent labeling
The double-labeling assay for detecting apoptotic cells and ghrelin-positive cells was carried out as described elsewhere.45 Briefly, after slides had been stained with TUNEL kit as described above, slides were washed three times in PBS for 10 min. In order to reduce nonspecific background, the sections were blocked with 2% sheep and horse sera in PBS for 30 min at room temperature. The sections were then incubated with the blocking solution containing the primary antibody, at 4 °C overnight in a moist box. Slides were washed three times in PBS for 10 min prior to addition of rhodamine-labeled antibodies (Jackson Immune Research Laboratories, West Grove, PA, USA). Nuclei were visualized by 10-min staining of 4′,6-diamidino-2-phenylindole (DAPI; dilution 1 : 2000; Sigma).The sections were mounted in 80% glycerol and examined with an inverted microscope (Axio Imager M1 microscope; Zeiss).
Statistical analysis
Results are presented as mean±S.E.M. from at least three independent experiments, and were analyzed for statistically significant differences using Student’s t-test. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed by using SPSS 15.0 software (IBM, Somers, NY, USA).
Abbreviations
- IR:
-
ionizing radiation
- LCs:
-
Leydig cells
- GCs:
-
germ cells
- GHS-R:
-
growth hormone secretagogue receptor
- T:
-
testosterone
- ROS:
-
reactive oxygen species
- SC:
-
Sertoli cells
- SCF:
-
stem cell factor
- D-GHRP:
-
[D-Lys-3]-GHRP-6
- ZBTB16:
-
zinc finger and BTB domain containing 16
- POU5F1:
-
POU domain, class 5, transcription factor 1
- SOHLH2:
-
spermatogenesis and oogenesis specific basic helix-loop-helix 2
- LDH-C4:
-
lactate dehydrogenase C4
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- LH:
-
luteinizing hormone
- FSH:
-
follicle-stimulating hormone
- MDA:
-
malondialdehyde
- Vit C/E:
-
vitamin C/E
- GPX:
-
glutathione peroxidase
- T-SOD:
-
total superoxide dismutase
- PFTα:
-
pifithrin-α
- DEDT:
-
diethyldithiocarbamic acid sodium salt trihydrate
- i.p.:
-
intraperitoneally
- HPG:
-
hypothalamic-pituitary-gonadal
- NLS:
-
nuclear localization signal
- DAPI:
-
4′,6-diamidino-2-phenylindole
References
Aly HA, Azhar AS . Methoxychlor induced biochemical alterations and disruption of spermatogenesis in adult rats. Reprod Toxicol 2013; 40: 8–15.
Wan HT, Mruk DD, Wong CK, Cheng CY . Targeting testis-specific proteins to inhibit spermatogenesis: lesson from endocrine disrupting chemicals. Expert Opin Ther Targets 2013; 17: 839–855.
Wang DH, Hu JR, Wang LY, Hu YJ, Tan FQ, Zhou H et al. The apoptotic function analysis of p53, Apaf1, Caspase3 and Caspase7 during the spermatogenesis of the Chinese fire-bellied newt Cynops orientalis. PLoS One 2012; 7: e39920.
Bujan L, Walschaerts M, Moinard N, Hennebicq S, Saias J, Brugnon F et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril 2013; 100: 673–680.
Maiorino M, Ursini F . Oxidative stress, spermatogenesis and fertility. Biol Chem 2002; 383: 591–597.
Turner TT, Lysiak JJ . Oxidative stress: a common factor in testicular dysfunction. J Androl 2008; 29: 488–498.
Rasoulpour RJ, Boekelheide K . NF-kappaB activation elicited by ionizing radiation is proapoptotic in testis. Biol Reprod 2007; 76: 279–285.
Xu G, Vogel KS, McMahan CA, Herbert DC, Walter CA . BAX and tumor suppressor TRP53 are important in regulating mutagenesis in spermatogenic cells in mice. Biol Reprod 2010; 83: 979–987.
Otala M, Suomalainen L, Pentikainen MO, Kovanen P, Tenhunen M, Erkkila K et al. Protection from radiation-induced male germ cell loss by sphingosine-1-phosphate. Biol Reprod 2004; 70: 759–767.
Garcia MC, Lopez M, Alvarez CV, Casanueva F, Tena-Sempere M, Dieguez C . Role of ghrelin in reproduction. Reproduction 2007; 133: 531–540.
Gaytan F, Barreiro ML, Caminos JE, Chopin LK, Herington AC, Morales C et al. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab 2004; 89: 400–409.
Barreiro ML, Gaytan F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E et al. Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol Reprod 2002; 67: 1768–1776.
Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE et al. Novel expression and functional role of ghrelin in rat testis. Endocrinology 2002; 143: 717–725.
Zhu CC, Zhang H, Zhang JS, Li Z, Zhao J, Li W et al. Inhibition of ghrelin signaling improves the reproductive phenotype of male ob/ob mouse. Fertil Steril 2013; 99: 918–926.
Tena-Sempere M . Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitam Horm 2008; 77: 285–300.
Kheradmand A, Dezfoulian O, Alirezaei M . Ghrelin regulates Bax and PCNA but not Bcl-2 expressions following scrotal hyperthermia in the rat. Tissue Cell 2012; 44: 308–315.
Barreiro ML, Gaytan F, Castellano JM, Suominen JS, Roa J, Gaytan M et al. Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger ribonucleic acid expression in rat testis. Endocrinology 2004; 145: 4825–4834.
Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, Pandolfi PP et al. Repression of kit expression by Plzf in germ cells. Mol Cell Biol 2007; 27: 6770–6781.
Barrios F, Filipponi D, Campolo F, Gori M, Bramucci F, Pellegrini M et al. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci 2012; 125 (Pt 6): 1455–1464.
Goldberg E, Eddy EM, Duan C, Odet F . LDHC: the ultimate testis-specific gene. J Androl 2010; 31: 86–94.
Shah FJ, Tanaka M, Nielsen JE, Iwamoto T, Kobayashi S, Skakkebaek NE et al. Gene expression profiles of mouse spermatogenesis during recovery from irradiation. Reprod Biol Endocrinol 2009; 7: 130.
Zhang S, Li W, Zhu C, Wang X, Li Z, Zhang J et al. Sertoli cell-specific expression of metastasis-associated protein 2 (MTA2) is required for transcriptional regulation of the follicle-stimulating hormone receptor (FSHR) gene during spermatogenesis. J Biol Chem 2012; 287: 40471–40483.
Li Y, Huang Y, Piao Y, Nagaoka K, Watanabe G, Taya K et al. Protective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testis. Reprod Biol Endocrinol 2013; 11: 23.
Coureuil M, Ugolin N, Tavernier M, Chevillard S, Barroca V, Fouchet P et al. Puma and Trail/Dr5 pathways control radiation-induced apoptosis in distinct populations of testicular progenitors. PLoS One 2010; 5: e12134.
Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med 2007; 176: 805–813.
Cetin E, Kanbur M, Cetin N, Eraslan G, Atasever A . Hepatoprotective effect of ghrelin on carbon tetrachloride-induced acute liver injury in rats. Regul Pept 2011; 171: 1–5.
Colpi GM, Contalbi GF, Nerva F, Sagone P, Piediferro G . Testicular function following chemo-radiotherapy. Eur J Obstet Gynecol Reprod Biol 2004; 113 (Suppl 1): S2–S6.
Rasoulpour T, DiPalma K, Kolvek B, Hixon M . Akt1 suppresses radiation-induced germ cell apoptosis in vivo. Endocrinology 2006; 147: 4213–4221.
Meistrich ML, Kangasniemi M . Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J Androl 1997; 18: 80–87.
Li T, Hu J, He GH, Li Y, Zhu CC, Hou WG et al. Up-regulation of NDRG2 through nuclear factor-kappa B is required for Leydig cell apoptosis in both human and murine infertile testes. Biochim Biophys Acta 2012; 1822: 301–313.
Zhang L, Tang J, Haines CJ, Feng HL, Lai L, Teng X et al. c-kit and its related genes in spermatogonial differentiation. Spermatogenesis 2011; 1: 186–194.
Hou Y, An J, Hu XR, Sun BB, Lin J, Xu D et al. Ghrelin inhibits interleukin-8 production induced by hydrogen peroxide in A549 cells via NF-kappaB pathway. Int Immunopharmacol 2009; 9: 120–126.
Xu Z, Lin S, Wu W, Tan H, Wang Z, Cheng C et al. Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-kappaB pathways and mitochondrial protective mechanisms. Toxicology 2008; 247: 133–138.
Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, Pilc K, Suchanek R, Pierzchala K et al. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol 2007; 58 (Suppl 1): 53–64.
El Eter E, Al Tuwaijiri A, Hagar H, Arafa M . In vivo and in vitro antioxidant activity of ghrelin: Attenuation of gastric ischemic injury in the rat. J Gastroenterol Hepatol 2007; 22: 1791–1799.
Aitken RJ, Roman SD . Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol 2008; 636: 154–171.
Yang B, Sun H, Li W, Zhu C, Jian B, Hou W et al. Expression of Rap1 during germ cell development in the rat and its functional implications in 2-methoxyacetic acid-induced spermatocyte apoptosis. Urology 2013; 81: 696 e691–696 e698.
Beumer TL, Roepers-Gajadien HL, Gademan IS, van Buul PP, Gil-Gomez G, Rutgers DH et al. The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ 1998; 5: 669–677.
Porteiro B, Diaz-Ruiz A, Martinez G, Senra A, Vidal A, Serrano M et al. Ghrelin requires p53 to stimulate lipid storage in fat and liver. Endocrinology 2013; 154: 3671–3679.
Velasquez DA, Martinez G, Romero A, Vazquez MJ, Boit KD, Dopeso-Reyes IG et al. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 2011; 60: 1177–1185.
Kaushal N, Bansal MP . Selenium variation induced oxidative stress regulates p53 dependent germ cell apoptosis: plausible involvement of HSP70-2. Eur J Nutr 2009; 48: 221–227.
Maheshwari A, Misro MM, Aggarwal A, Sharma RK, Nandan D . Pathways involved in testicular germ cell apoptosis induced by H2O2 in vitro. FEBS J 2009; 276: 870–881.
Zhang S, Zeng Y, Qu J, Luo Y, Wang X, Li W . Endogenous EGF maintains Sertoli germ cell anchoring junction integrity and is required for early recovery from acute testicular ischemia/reperfusion injury. Reproduction 2013; 145: 177–189.
Liang Y, Dong Y, Zhao J, Li W . YES1 activation elicited by heat stress is anti-apoptotic in mouse pachytene spermatocytes. Biol Reprod 2013; 89: 131.
Ray SK, Schaecher KE, Shields DC, Hogan EL, Banik NL . Combined TUNEL and double immunofluorescent labeling for detection of apoptotic mononuclear phagocytes in autoimmune demyelinating disease. Brain Res Brain Res Protoc 2000; 5: 305–311.
Acknowledgements
We are grateful to Hui Wang (Department of Foreign Language, Fourth Military Medical University, China) for her careful assistance during the language editing of the manuscript. This work was supported by the Natural Science Foundation of China (NSFC): 31271248, 81271195 and 81371446.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Agostini
Supplementary Information accompanies this paper on Cell Death and Disease website
Supplementary information
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Li, W., Zeng, Y., Zhao, J. et al. Upregulation and nuclear translocation of testicular ghrelin protects differentiating spermatogonia from ionizing radiation injury. Cell Death Dis 5, e1248 (2014). https://doi.org/10.1038/cddis.2014.223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2014.223
Keywords
This article is cited by
-
Fecal Microbiota Transplantation-Mediated Ghrelin Restoration Improves Neurological Functions After Traumatic Brain Injury: Evidence from 16S rRNA Sequencing and In Vivo Studies
Molecular Neurobiology (2024)
-
Testicular STAC3 regulates Leydig cell steroidogenesis through potentiating mitochondrial membrane potential and StAR processing
Cell and Tissue Research (2021)
-
Ghrelin alleviates endoplasmic reticulum stress and inflammation-mediated reproductive dysfunction induced by stress
Journal of Assisted Reproduction and Genetics (2019)
-
Interference with lactate metabolism by mmu-miR-320-3p via negatively regulating GLUT3 signaling in mouse Sertoli cells
Cell Death & Disease (2018)
-
Unexpected requirement for a binding partner of the syntaxin family in phagocytosis by murine testicular Sertoli cells
Cell Death & Differentiation (2016)