Abstract
NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2+ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2+ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2+ cells to give rise to Bergmann glia and astrocytes remains unchanged. Overall, our study reveals a novel Bergmann glia fate of Olig2/Plp-positive NG2 progenitors, demonstrates the differentiation of these progenitors into various functional glial cell types, and provides significant insights into the fate and function of Olig2/Plp-positive progenitor cells in health and disease.
Similar content being viewed by others
Main
NG2 (nerve/glial antigen2) is a chondroitin sulfate proteoglycan. Progenitor cells that express NG2 (termed ‘NG2+ cells’) constitute 5–8% of cells in the central nervous system,1, 2 but their fate and function in health and disease still remain controversial. These progenitor cells can be identified by the expression of several additional molecular markers, including platelet-derived growth factor receptor-α (PDGFRα) and Olig2 proteins, and the proteolipid (Plp) promoter. NG2+ cells have been classified as oligodendrocyte precursor cells (OPCs), although they can also generate type-2 astrocytes in vitro.3, 4 Accumulating evidence using various lines of genetic cell-fate mapping mice reveals multiple fates of NG2 cells in vivo. These include interneurons,5, 6, 7 principal neurons,8, 9, 10 and astrocytes.10, 11, 12, 13 However, a recent report concluded that the only fate for NG2+ cells is to become oligodendrocytes.14
In the present study, we employed both tamoxifen-inducible Plp15 and Olig2,16, 17, 18 the Cre transgenes to ascertain the fates of NG2+ cells in the pre- and postnatal cerebellum, and found that, in addition to oligodendroglia, they give rise to astroglia after prenatal tamoxifen administration, and to Bergmann glia (BG) after postnatal tamoxifen administration. Thus, cerebellar NG2+ cells are multipotential. Furthermore, we employed inhibitors of ionotropic glutamate receptors to evaluate whether glutamate signaling is involved in NG2+ cell-fate/differentiation pathways by tracing the fates of the extended yellow fluorescent protein (EYFP)-positive cells, and found evidence suggesting that glutamate receptors play a role in the normal development of BG. We also reveal that, despite a reactive response to injury, the multiple lineage potential of NG2+ cells remains unchanged in the cerebellum in a hypoxic–ischemic model of neonatal brain injury.19, 20 Thus, our work reveals a novel BG fate of Olig2/Plp-positive NG2 progenitors, and provides important insights into the fate and function of the NG2+ progenitors in health and disease.
This study explores a controversial area and defines the developmental potential of the NG+ cells of the cerebellum. Previous studies have reported a number of divergent results on the fates of NG2+ cells. Our work provides strong weight to the view that NG2+ cells readily generate oligodendrocytes as well as astrocytes, but do not generate neurons. Our study also makes the crucial point that the outcomes of the NG2+ cell-fate mapping experiments can be markedly different, depending upon the time point at which Cre-dependent labeling is initiated, and the surprising result is that NG2+ cells can give rise to BG late in cerebellar development.
Results
Plp-expressing NG2+ cells primarily generate oligodendrocytes in the cerebellum
The Plp gene encodes an integral membrane proteolipid protein and its smaller isoform DM-20, which together constitute >50% of the total protein in CNS myelin. Postnatal Plp promoter activity is largely confined to oligodendroglial lineage cells.21, 22, 23, 24 To examine NG2+ cell fates in the developing mouse cerebellum, we used Plp-Cre-ERT2/Rosa26-EYFP (PCE/R) double-transgenic mice9, 13 to identify tamoxifen-inducible cerebellar expression of Plp-positive progenitor cells and their progeny.
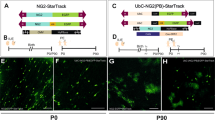
When tamoxifen was injected at postnatal day 7 (P7), strong EYFP immunoreactivity was observed throughout the P60 cerebellum (Figure 1a). Specific EYFP immunoreactivity was shown in the molecular and the Purkinje cell layers (Figures 1 a and a'). Earlier tamoxifen injection at embryonic day 19.5 (E19.5) exhibited a similar expression pattern (Figure 1b). Diffuse EYFP-positive cells were observed throughout the Purkinje cell and the molecular layers (Figure 1b). A number of EYFP-positive cells were also observed in the white matter track (Figures 1c and d). In the white matter, many of these EYFP-positive cells expressed immature oligodendrocyte marker, NG2 (Figures 1e–g; ∼32.6% (127/390, tamoxifen injection (t.i.) at E19.5, analyzed at P8), ∼22.3% (194/869, t.i. at E19.5, analyzed at P15), and ∼16.6% (114/687, t.i. at P7, analyzed at P60) were double labeled (n=6 mice/group)).
Expression pattern of Plp-expressing NG2+ cells in the mouse cerebellum: Plp-expressing NG2+ cells primarily generate oligodendrocytes. (a) Sagittal sections of the postnatal day 60 (P60) Plp-Cre-ERT2/Rosa26-EYFP (PCE/R) double-transgenic mouse cerebellum immunofluorescence stained with anti-GFP after tamoxifen injection (t.i.) at P7. Strong and specific EYFP-positive cells were observed throughout the cerebellum. (a') A higher magnification view of a rectangle area in (a). (b) Sagittal sections of the PCE/R double-transgenic mouse cerebellum immunofluorescence stained with anti-GFP. Tamoxifen was injected at embryonic day 19.5 (E19.5) and sections analyzed at P15. Specific immunoreactivity encompassing both somata and cellular processes was seen in the molecular and the Purkinje cell layers. (c and d): EYFP-positive cells were present in the white matter of the cerebellum at P60 (c) and P15 (d). (e and f) Double immunofluorescence with anti-GFP and the immature oligodendrocyte marker, anti-NG2 showed a colocalization in the white matter of the cerebellum. (g) Quantification of the percentile of EYFP-positive cells immunoreactive for NG2. As the distribution of oligodendrocyte lineage cells was concentrated in the white matter region of the cerebellum, the counting was performed in the white matter. (h and j): P15 (h) and P60 (j) PCE/R cerebellar sagittal sections were double immunofluorescence stained with anti-GFP and anti-O1. Tamoxifen was injected at E19.5 or P7, respectively. (i and k): P15 (i) and P60 (k) cerebellar sagittal sections were double immunofluorescence stained with anti-GFP and anti-Olig2. Tamoxifen was injected at E19.5 or P7, respectively. (l and m): P15 (l) and P60 (m) cerebellar sagittal sections were double immunofluorescence stained with anti-GFP and anti-PDGFRα. Tamoxifen was injected at E19.5 or P7, respectively. (n) Quantification of the percentile of EYFP-positive cells in the white matter that were immunoreactive for Olig2, O1, or PDGFRα. Abbreviations: CN, cerebellar nuclei; gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; PPEP, Plp-expressing progenitor cells; t.i., tamoxifen injection; wm, white matter. Scale bars: (a and b)=150 μm; (a', c, and d)=50 μm; (f, e, k, h–m)=25 μm
To further examine whether Plp-expressing NG2+ progenitor cells differentiate into oligodendrocytes, t.i. was given at E19.5 or P7, and sections from P15 or P60, respectively, were examined by double immunohistochemistry with several oligodendroglial lineage markers, including the pan-lineage marker Olig2 (Figures 1i and k), the oligodendrocyte precursor marker PDGFRα (Figures 1l and m), and a marker for both immature and mature oligodendrocytes, O1 (Figures 1h and j). Immunofluorescence staining showed that a large proportion of EYFP-positive cells were colabeled with Olig2 (Figures 1i and k). The extent to which Plp-expressing NG2+ cells gave rise to oligodendrocytes varied in different cerebellar areas we assessed. In the white matter, double-immunofluorescence labeling with anti-EYFP and anti-Olig2 revealed that ∼66% (193/293, t.i. at E19.5, analyzed at P15) and ∼78% (184/237, t.i. at P7, analyzed at P60) of EYFP+ cells expressed Olig2 (Figures 1i, k) (n=6 mice). A subset of EYFP-positive profiles coexpressed O1 (Figures 1h, j, ∼57% (98/172, t.i. at E19.5, analyzed at P15) and ∼63% (151/241, t.i. at P7, analyzed at P60) were double labeled). However, PDGFRα-positive profiles were relatively few (Figures 1l, m; ∼18% (21/120 t.i. at E19.5, analyzed at P15) and ∼1% (1/98, t.i. at P7, analyzed at P60) were double labeled; n=6 mice). Consistent with previous findings on NG2+ cell fates, these data indicate that NG2+ cells give rise primarily to oligodendrocytes.
Plp-expressing NG2+ cells also give rise to astrocytes and BG in the cerebellum
NG2+ progenitor cells can generate oligodendrocytes or type-2 astrocytes in vitro, depending on the composition of the culture medium.3 We examined EYFP-positive cells for glial fibrillary acidic protein (GFAP) expression in the cerebellum of the PCE/R mice in which tamoxifen was administered at E19.5 and cerebellar sections analyzed at P15. Consistent with previous data analyzed in the forebrain using the same Plp-Cre mouse line,9, 13 many of the EYFP-positive cells in the granular layer expressed GFAP (Figures 2a and 3a), indicating that Plp-expressing NG2+ cells gave rise to astrocytes in the cerebellum. Surprisingly, later t.i. revealed a dramatic profile of EYFP-positive BG throughout the cerebellum (Figures 2b and h and 3b). BG were not labeled to the same extent with fetal administration of tamoxifen. In addition, the number of EYFP+ BG was significantly increased at P60 (t.i. at P7) compared with P15 (t.i. at E19.5; P<0.01; Figure 3b). BG are specialized glia in the cerebellum that are located in proximity to the soma of Purkinje cells, and extending their processes through the molecular layer to terminate at the pial surface.25 To confirm the localization of EYFP to BG, double immunostaining was performed with several cell type-specific markers. Double staining with the pan-neuronal marker NeuN revealed that EYFP immunoreactivity was not present in neurons (Figure 2b and 3c), and staining with the pan-oligodendroglial marker Olig2 indicated that these EYFP+ cells were not oligodendroglia at all postnatal ages (P15 was shown as an example; Figure 3d). Double staining was performed to label calbindin binding protein (CaBP), a specific marker for all adult Purkinje cells,26, 27, 28 together with EYFP. EYFP-positive cells were CaBP-negative (Figure 3e). These results clearly indicate that Plp-expressing NG2+ cells give rise to astrocytes and BG in the mouse cerebellum in an age-dependent manner.
Plp-expressing NG2+ cells also give rise to astrocytes and Bergmann glia (Part 1). (a) Double-immunofluorescence labeling of P15 sections with anti-GFP and anti-GFAP in the PCE/R mouse cerebellum showed that EYFP+ cells were able to give rise to astrocytes. Tamoxifen was injected at E19.5. (a' and a'') represent the rectangular area of (a). (b) Double immunofluorescence with anti-GFP and the pan-neuronal marker anti-NeuN showed no colocalization in the molecular and the Purkinje cell layers of the cerebellum. (c and d) Double immunostaining with anti-GFP and anti-GFAP (red) showed that EYFP+ cells gave rise to astrocyte and Bergmann glia at P15 cerebellum (t.i. at E19.5). (e) EYFP+ Bergmann glia was obvious in the lateral part of cerebellar lobule X. (e' and e'') represent the rectangular area of (e). (f and h) Double immunofluorescence staining with anti-GFP and anti-GFAP showed that EYFP+ Bergmann glia expressed GFAP. (g) EYFP+ Bergmann glia was more abundant when tamoxifen was injected at P7 and sections analyzed at P60. Abbreviations: gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; PPEP, Plp-expressing progenitor cells; t.i., tamoxifen injection; wm, white matter. Scale bars: (a)=50 μm; (b)=150 μm; (e)=250 μm; (f and h)=100 μm; (g)=125 μm
Plp-expressing NG2+ cells also give rise to astrocytes and BG (Part 2). (a and b) Quantification of the percentile of EYFP+GFAP+ cells out of the whole GFAP+ cells in the white matter. The number of EYFP+ BG glia was significantly increased at P60 (t.i. at P7) compared with P15 (t.i. at E19.5) (**P<0.01) (b) (c) Double immunostaining with anti-GFP and anti-NeuN at later tamoxifen injection (at P7) and analysis (at P60) showed that GFP-positive BG were not neurons in the cerebellum. (d) Sagittal sections double immunofluorescence stained with anti-GFP and anti-Olig2. Olig2 immunoreactivity was not observed in EYFP-positive BG, but present in EYFP-positive cells in the granular layer. (e) The Purkinje cell marker calcium binding protein (CaBP) staining at P15 showed no specific EYFP expression in the Purkinje cells in the cerebellum. Abbreviations: AC, astrocyte; BG, Bergmann glia; gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; t.i., tamoxifen injection; wm, white matter. Scale bars: (c and d)=100 μm; (e)=25 μm
Olig2-expressing NG2+ cells differentiate into oligodendrocytes, astrocytes, and BG, but not neurons
To corroborate the results on the fates of the Plp-expressing NG2+ cells, and to further confirm that NG2 progenitor cells could give rise to multiple cell types in the cerebellum, we next generated transgenic Olig2-CreERT2/Rosa26-EYFP (OCE/R) mice. Olig2 is a basic helix-loop-helix (bHLH) transcription factor that is expressed in NG2+ progenitor lineage cells from embryonic to adult stages.16, 17, 18 Strong and specific EYFP immunoreactivity was observed throughout the cerebellum (Figures 4a and b). A number of EYFP-positive cells were observed in the white matter (Figures 4a and b). Double immunofluorescence labeling of EYFP and the oligodendrocyte lineage marker O1 or PDGFRα revealed that ∼77% (135/175, t.i. at P6, analyzed at P11) of EYFP+ cells expressed O1, whereas ∼16% (35/217, t.i. at P6, analyzed at P11; n=6 mice) of EYFP+ cells expressed PDGFRα. These data further confirm that the majority of EYFP-positive cells in cerebellar white matter give rise to oligodendrocytes (Figures 4c and d).
Olig2-expressing NG2+ cells primarily generate oligodendrocytes in the mouse cerebellum. (a and b) Single immunofluorescence labeling for anti-GFP in the Olig2-Cre-ER/Rosa26-EYFP (OCE/R) mouse cerebellum. Tamoxifen was injected at P5 and sections analyzed at P8 (a). Tamoxifen was injected at P6 and analyzed at P11 (b). In both cases, strong EYFP-positive cells were observed throughout the cerebellum. (b' and b'') High-power images of the rectangular region of (b). EYFP-positive Bergmann glia-like cells were obvious in the molecular and the Purkinje cell layers of the cerebellum (b'). There were abundant EYFP-positive profiles in the white matter (b''). (c and -d) Double immunofluorescence labeling with anti-GFP (green) and anti-O1 (red) showed that some EYFP-positive cells coexpressed O1. Quantification of the percentile of O1+ and PDGFRα+ oligodendrocyte lineage cells in the white matter (d). Abbreviations: gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; t.i., tamoxifen injection; wm, white matter. Scale bars: (a and b)=100 μm; (c)=25 μm
We next examined whether Olig2-expressing NG2+ progenitor cells could also give rise to astrocytes and BG in the cerebellum. Immunostaining of a transverse section revealed that anti-GFAP immunoreactivity was present in the somata and processes of astrocytes (Figures 5a and h) and BG (Figures 5b,d ) in the granular layer. The percentile of EYFP+ astrocytes and BG was dependent on the age of the mice. P8 cerebellum (t.i. at P5) showed ∼6% (16/276) astrocytes and ∼16% (27/172) BG of EYFP+ cells, whereas P11 cerebellum (t.i. at P6) revealed ∼13% (39/302) astrocytes and ∼29% (61/211) BG of EYFP+ cells (P<0.05; n=6 mice). Thus, consistent with our Plp-Cre fate mapping data, these results further confirm that that Olig2-expressing NG2+ cells can give rise to astrocytes and BG in the mouse cerebellum.
Olig2-expressing NG2+ cells also give rise to astrocytes and BG. (a–d) Double immunofluorescence labeling of P11 sections with anti-GFP and anti-GFAP in the OCE/R mouse cerebellum. There were double-labeled cells immunopositive for EYFP and GFAP confirming that they were astrocytes (a and a'). Tamoxifen was injected at P6. (a') A higher magnification and separate view of the region indicated in (a). (b–d) Double immunostaining with anti-GFP (green) and anti-GFAP (red) showed that Olig2-Cre expression was present in BG at the P11 cerebellum. (e–g) Double immunofluorescence labeling of P11 sections with anti-GFP and anti-Olig2 showed that Olig2+ cells were not colabeled with EYFP+ BG. (h and i) Quantification of the percentile of EYFP+ and GFAP+ astrocytes (h) and BG (i) out of the whole GFAP+ cells (*P<0.05). Abbreviations: AC, astrocyte; BG, BG; gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; t.i., tamoxifen injection. Scale bars: (a, b, and e)=100 μm
NG2+ cells do not give rise to neurons in the mouse cerebellum
Whether NG2+ cells can give rise to neurons is controversial. To determine whether Olig2-expressing NG2+ cells generate EYFP-positive neurons, we co-immunostained for EYFP and NeuN. We did not find any EYFP+/NeuN+ cells throughout the cerebellum of these mice (Figures 6a–c). Further analysis confirmed the lack of EYFP+/NeuN+ neurons at different ages of the mice and time points we examined, and we did not find any ectopic expression of Olig2-driven EYFP in the OCE/R mice 24 h after tamoxifen treatment.
NG2+ cells do not give rise to neurons in the OCE/R mouse cerebellum, and a very small subset of cerebellar nuclei neurons in the PCE/R mouse cerebellum ectopically express EYFP. (a–c) Immunofluorescence labeling with the neuronal marker NeuN revealed that EYFP-immunopositive cells did not express NeuN in the granular layer and in the white matter of the OCE/R cerebellum. (d–f) Sagittal sections of P15 PCE/R mouse cerebellum immunofluorescence stained with anti-GFP and anti-NeuN after t.i. at E19.5. A few EYFP-positive cells were colabeled with anti-NeuN representing a very small subset of ectopically EYFP-labeled cerebellar nuclei neurons. (g) Double immunofluorescence labeling of P60 sections with anti-GFP and anti-NeuN in PCE/R mouse cerebellum showed no double-labeled cells. (h and i) Ectopic EYFP staining 24 h after tamoxifen injection in the PCE/R mouse cerebellum. (h) Similar to those seen in (d–f), ectopic expression of few EYFP+ neurons could only be found in the cerebellar nuclei areas. (i) No ectopic EYFP expression in any cell types in the cortex and white matter of the cerebellum. Abbreviations: gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; t.i., tamoxifen injection; wm, white matter. Scale bars: (b, d–h)=50 μm; (i)=125 μm
We next sought to investigate whether Plp-expressing NG2+ cells could generate EYFP-positive neurons in the mouse cerebellum of PCE/R mice. We immunostained for EYFP and NeuN in the cerebellum of P15 (t.i. at E19.5) and P60 (t.i. at P7) PCE/R mice. When tamoxifen was injected at E19.5, a very small number of EYFP+ cells expressed NeuN, a pan-neuronal marker, and they could only be found in the cerebellar nuclei region (∼0.7%, 5/683; Figures 6d–f). We did not find any EYFP+/NeuN+ neurons in any other areas of the cerebellum, and thus the actual percentile of NeuN+ neurons in all EYFP+ cells was very small. In addition, we found that a very small number of EYFP+/NeuN+ neurons were already apparent 24 h after tamoxifen treatment (Figure 6h), and again those few neurons ectopically expressing EYFP were only found in the cerebellar nuclei region (Figures 6h and i), indicating that they must have been already neurons or nearly differentiated neurons that happened to express the Plp promoter at the time of tamoxifen treatment. These results indicate that NG2+ cells do not give rise to neurons in the mouse cerebellum.
Glutamate receptor antagonist treatment generally does not alter the fates of NG2+ cells, but the development of BG is impaired
Given the BG fate of NG2+ progenitors, we next sought to examine possible mechanisms of the cell-fate control in the mouse cerebellum. Given the previously known role of glutamate-triggered calcium signaling in shaping the BG cell fate in mice,29, 30 we used the N-Methyl-D-aspartate (NMDA) glutamate receptor antagonist dizocilpine maleate (MK-801) and the non-NMDA glutamate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f) quinoxaline-2,3-dione (NBQX) to evaluate the effects of ionotropic glutamate receptor signaling on the generation of BG from NG2+ cells in PCE/R mice. Tamoxifen was injected at P0 followed by subsequent MK-801 or NBQX treatment. We found that the general distribution profile and pattern of EYFP-positive cells were overall indistinguishable between the control (Figure 7a) and NBQX treatment (Figure 7b). Normal development of EYFP-positive BG was somewhat impaired (Figure 7d) compared with saline-treated control (Figure 7c). In the control group, EYFP-positive radial processes corresponding to those of BG were densely arranged within the cortex (Figures 7c and c'). Most of the processes extended from the somata to pial boundary of surface, which were aligned in the Purkinje cell layer (Figures 7c and c). However, MK-801-treated EYFP-positive BG processes were not aligned in the cortex and did not reach the pial surface (Figures 7d and d'). In addition, when analyzed at P24, the number of EYFP+ BG was significantly decreased in MK-801-treated group compared with the control (Figures 7h and i, P<0.001). These results indicate that glutamate receptor antagonist treatment generally did not alter NG2+ cell fates, but NMDA glutamate receptors does appear to play a role in normal development of BG.
MK-801 treatment impairs the normal development of BG. (a and b) Single immunofluorescence labeling with anti-GFP in the PCE/R mouse cerebellum after treatment of NBQX. Tamoxifen was injected at P0 and sections analyzed at P24. General distribution profile and pattern of EYFP-positive cells were normal for both the control (a) and NBQX treatment (b). (c and d) Single immunofluorescence labeling with anti-GFP in the PCE/R mouse cerebellum after MK-801 treatment. Tamoxifen was injected at P0 and sections analyzed at P13 and P24. The general morphology of EYFP-positive BG was impaired (d) compared with the control (c). (e–g) The number of EYFP+ BG was significantly decreased in the MK-801 treatment group (f and g). (h and i) Quantification of the number of EYFP+/GFAP+ BG after NBQX or MK-801 treatment (***P<0.001). Abbreviations: BG, Bergmann glia; gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; t.i., tamoxifen injection. Scale bars: (a and b)=100 μm; (c–g)=125 μm
Hypoxic–ischemic brain injury does not alter the cell fate of NG2+ cells, but the number of oligodendroglial cells is increased
NG2+ cells proliferate in response to injury,1 and previous studies have suggested that injury or disease can reprogram NG2+ cell fates.31, 32, 33 We examined whether fate alterations of NG2+ progenitor cells could occur in our established hypoxic–ischemic model of neonatal brain injury in mice.19, 20 In this mouse model, which reliably resembles the pathology of periventricular leukomalacia (PVL) in premature infants, NG2+ progenitor cells are a major substrate of the disease. In addition, PVL injures the cerebellum as well as the telencephalon.34, 35, 36, 37, 38 We thus investigated whether PVL-like injury might alter the fates of Olig2-expressing NG2+ cells. Hypoxic–ischemic brain injury was induced in P6 OCE/R mouse pups, and cerebellar sections were analyzed at P11. In the hypoxic–ischemic cerebellum, the basic topography of EYFP-positive cells was not significantly changed (Figures 8a–d). Next, immunostaining for myelin basic protein (MBP) was performed to evaluate the severity of white matter injury. MBP-positive oligodendrocyte/myelin formation did not appear to be affected in the cerebellum (Figures 8c–f). In addition, neither the number of EYFP+GFAP+ astrocytes and BG (Figure 8i) nor basic topography of these cells (Figures 8a) was different between the control and hypoxic–ischemic mice. However, we found that the number of EYFP+O1+ oligodendroglial cells in the cerebellum was increased in response to hypoxic–ischemic injury in the PVL mice compared with the controls (Figures 8g, h, P<0.01), likely because of a reactive response to hypoxia–ischemia.
Hypoxic–ischemic injury does not result in fate changes in the OCE/R mouse cerebellum, but the number of oligodendrocyte lineage cells is increased. (a–f) Immunofluorescence labeling of EYFP and MBP in the OCE/R mouse cerebellum after induction of neonatal brain injury using a mouse model of PVL. Tamoxifen was injected at P0 and sections analyzed at P11. The number and anatomical topography of EYFP+ cells were not significantly changed between the control (a) and the hypoxic–ischemic cerebellum (b). The topography of MBP-positive oligodendrocytes in the cerebellum was relatively normal after hypoxic–ischemic injury (c and e: control; d and f: PVL). (g and h) Double immunostaining with anti-GFP and anti-O1 showed abundant double-labeled cells in the granular layer. (i) Quantification of the percentile of EYFP+ cells that expressed GFAP+ for astrocytes and BG, PDGFRα for oligodendroglial precursor cells, and O1 for oligodendroglial cells in the cerebellum after PVL induction. The number of EYFP+/O1+ oligodendroglial cells was increased after PVL induction (**P<0.01). Abbreviations: AC, astrocyte; BG, Bergmann glia; Ctrl, control; gl, granule cell layer; ml, molecular layer; pcl, Purkinje cell layer; PVL, periventricular leukomalacia; t.i., tamoxifen injection; wm, white matter. Scale bars: (a–d)=100 μm; (h and g)=125 μm; (j and i)=250 μm
Discussion
The fate of NG2+ cells has been a controversial issue for decades.39 In addition, previous studies have largely, if not exclusively, focused on examining NG2+ cell fates in the forebrain and spinal cord. In the present study, we have focused our attention on the cerebellum. Taking advantage of Plp and Olig2 promoter activity in NG2+ cells to drive expression of a tamoxifen-inducible Cre transgene, we generated PCE/R and OCE/R double-transgenic mice. Consistent with previous findings on NG2+ cell fates, we observed abundant oligodendrocyte differentiation in these mice. Thus, NG2+ cells give rise primarily to oligodendrocytes. Surprisingly, however, we discovered that a significant fate of the NG2 progenitors is BG in both the PCE/R and OCE/R mouse cerebellum. We also searched among the EYFP-positive cells for evidence of astrocytes or neurons. We observed a portion of the cells bearing the astrocyte marker GFAP and the characteristic astrocytic morphology in both the Plp-Cre and the Olig2-Cre mice. We did not observe EYFP-positive neurons in the OCE/R mice, indicating that NG2 progenitors do not give rise to neurons. We did, however, spot the occasional NeuN-positive neurons expressing EYFP in the PCE/R mouse cerebellum, but these cells were extremely rare and only found in the cerebellar nuclei region. We showed that those few EYFP+ neurons were not new neurons arising from NG2+ progenitors, because they were apparent 24 h after tamoxifen treatment, yet it should take weeks or longer for neurons to be derived from NG2+ cells. Therefore, those few neurons ectopically expressing EYFP must have been already neurons or nearly differentiated neurons that happened to express the Plp promoter at the time of tamoxifen treatment. However, the EYFP+ labeling of astrocytes and BG clearly was not ectopic. We showed that the ectopic expression of EYFP 24 h after t.i. was only seen in the few neurons in the cerebellar nuclei region (Figure 6h), and not in any other regions and cell types including astrocytes and BG in the PCE/R mouse cerebellum (Figure 6i), and there was no any ectopic expression of EYFP in the OCE/R mouse cerebellum.
Raff et al.3 indicated that OPCs could generate either oligodendrocytes or type-2 astrocytes in cultures of rat optic cells. More recently, fate mapping techniques using genetically engineered Cre–loxP system have enabled us to examine the fates of NG2+ cells directly in vivo. Using NG2-Cre BAC transgenic mice to trace the progeny of NG2 glia, Zhu et al.11, 12 demonstrated that a large number of fate-mapped astrocytes were found in the gray matter of the ventral forebrain and spinal cord. In addition, a number of studies indicated that NG2+ cells generate neurons as well as oligodendrocytes and astrocytes.4, 9, 40 Kondo and Raff4 showed that OPCs could revert into neural stem cells that could then give rise to neurons, oligodendrocytes, and astrocytes. Further studies supported this idea. In CNP-EGFP mice, NG2-expressing cells generated neurons as well as oligodendrocytes, both in culture5 and after being grafted into neurogenic areas of the embryonic or postnatal host brain.6, 7 Using Cre–Lox fate mapping studies, others further suggested that NG2 glia could give rise to neurons in the forebrain subventricular zone (SVZ) and hippocampus.9, 13, 40 NG2+ cells in the neocortex and piriform cortex expressed doublecortin, a marker of migratory neuronal progenitors in the forebrain.40 Some NG2+ cells in the piriform cortex expressed Sox2 and Pax6,9, 40 two neural stem cell markers. However, the neuronal fate of NG2+ cells was not observed by other studies using the NG2 or PDGFRα antibody to label SVZ or hippocampal stem cells or to detect NG2 or PDGFRα promoter activity in these stem cell populations in BAC transgenic mice.8, 11, 14, 41 Zhu et al.11, 12 reported that no neurons were generated from NG2 progenitors in constitutive NG2–Cre mice. In addition, no YFP-positive neurons were found in Olig2-Cre/Rosa YFP mice.42
Several factors contribute to the controversies regarding NG2 cell fates. First, the expression of PDGFRα, NG2, Olig2, or Plp are not exclusive in NG2+ cells, making NG2+ cell-fate mapping less straightforward, and if the targeted genes were not specific to NG2+ cells at the time of t.i., the integrity of the genetic cell-fate tracing experiments would be compromised. Therefore, checking for the reporter EYFP or Cre ectopic expression in the cerebellum shortly (e.g., 24 h) after t.i. was critical for the fate mapping studies. Moreover, the rarity and anatomical location of EYFP-positive neurons could make them difficult to detect. In our Plp-Cre lineage tracing experiments, very few EYFP-positive neurons were observed only in the cerebellar nuclei region, and we demonstrated that they were ectopically labeled neurons and were not directly derived from NG2+ cells. In addition, we sought to obtain consistent data using two different lines of transgenic mice and to double confirm results for each other. In addition, we showed that Cre expression was specific to NG2+ cells at the time points of t.i., indicating that NG2+ cells generate BG and astrocytes in the mouse cerebellum. We found that cerebellar NG2+ cells gave rise to astroglia after prenatal tamoxifen administration, and to BG after postnatal tamoxifen administration, in an age-dependent manner.
Regarding the development of BG, a previous report showed that GLAST-expressing cells in the external granular layer were present as early as E14 in the embryonic cerebellum.25 Cells expressing tenascin or GLAST had increased further and densely located beneath the multicellular layer of Purkinje cells by E18.43, 44, 45 During the first postnatal week when Purkinje cells establish monolayer alignment, cells expressing tenascin or GLAST are further compacted to form an epithelium-like lining in the Purkinje cell layer. Our study demonstrated that later P7 t.i. labeled more BG than earlier E19.5 t.i., suggesting that BG generation in the cerebellum is predominately postnatal.
After having revealed a previously unrecognized BG fate of NG2+ progenitors, we then examined the mechanisms of the cell-fate control in the cerebellum. Given the known role of glutamate-triggered calcium signaling in shaping the BG cell fate in mice, we used ionotropic glutamate receptor (NMDA versus non-NMDA type) antagonists to study the involvement of glutamate signaling in the regulation of the BG fate of NG2+ progenitors, and found that NMDA-type glutamate receptors play a role in the normal development of BG. Karadottir et al.46 documented oligodendroglial NMDA receptors in the cerebellum, although recent studies47, 48 emphasized that these receptors are few in number and that their deletion does not affect oligodendrocyte development. Interestingly, within this context, our results described here would instead suggest that these NMDA receptors might modulate development of BG in the cerebellum.
In addition, NG2+ cells are known to proliferate in response to injury,1 and studies have suggested that following trauma, NG2+ cells give rise to astrocytes as well as microglia32 and Schwann cells.33 Furthermore, NG2+ cells proliferate and generate astrocytes in ALS.31 We examined whether there might be alterations in the fates of NG2+ progenitor cells in a hypoxic–ischemic model of neonatal brain injury.19, 20 In this model, where NG2+ progenitor cells are a major substrate of the pathology, we found that, despite a reactive response of the O1+ oligodendroglial cells to injury, the fates of the NG2-expressing cells remain unchanged, with a similar multipotential capacity of giving rise to oligodendrocyte lineage cells, BG, and astrocytes in the cerebellum. Thus, the multipotential capacity of the NG2+ progenitor cells remains the same in health and disease.
Overall, by revealing a novel BG fate of Olig2/Plp-positive NG2 progenitors, our findings reported here represent a conceptual advance. Our study provides important insights into not only the differentiation of NG2+ cells into various functional glial cell types in the mouse cerebellum but also more generally in the functional role of the progenitor cells in health and disease.
Materials and Methods
Transgenic mice
All animal procedures conformed to institutional regulations of the University of California at Davis and guidelines of the NIH. Mice were housed in animal facilities of the University of California at Davis with a 12-h light/dark cycle and free access to food and water. The Plp-CreERT2 mice15 and Rosa26-EYFP reporter line49 were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained in the C57BL/6 background. These two lines were crossed to obtain PCE/R double-transgenic mice. In PCE/R mice, Cre-ERT2 fusion protein is expressed in the cytosol under the control of Plp promoter. After binding with tamoxifen, Cre recombinase translocates into the nucleus to mediate Cre–loxP recombination, thus eliciting permanent expression of the EYFP reporter gene in the Plp promoter-driven EYFP-expressing progenitors and their progenies. Olig2-Cre-ER transgenic mice18 were crossed to Rosa26-loxP-STOP-loxP-EYFP reporter transgenic mice49 to yield OCE/R transgenic mice, which carried a heterozygous Cre transgene and homozygous reporter transgenes. All mice were maintained on the C57BL/6 background. Both males and females were used in this study.
Mouse model of periventricular leukomalacia
A model of neonatal brain injury, which reliably resembles the neuropathology of PVL,19, 20 was induced in P6 mouse pups using ischemia induced by unilateral carotid ligation (UCL) and followed by hypoxia, which resulted in selective injury to the periventricular white matter. In brief, mice were anesthetized under ice (indirect cooling) and then underwent UCL followed by a 1-h recovery interval during which the pups were housed with the dam and kept on a thermal blanket to maintain body temperature at 33–34 °C. Next, the pups were placed in an airproof isolation chamber with 6.0% O2 for 35 min. After exposure to hypoxia, pups were housed with the dam for intervals of 24, 48, 72, or 96 h, as required for further experiments.
Drug treatments
Tamoxifen (T5648; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in an ethanol/sunflower seed oil (1 : 19) mixture at a concentration of 10 mg/ml. At P7, PCE/R pups were injected twice, 6–8 h apart intraperitoneally with tamoxifen (75 μg/g body weight). Glutamate receptor antagonist MK-801 (Sigma, St. Louis, MO, USA) at 1 mg/kg or NBQX (Tocris Cookson, St. Louis, MO, USA) at 30 mg/kg was given by intraperitoneal injection at P0 with t.i. Lesioned control animals were injected with PBS. These mice were killed 4 days after drug treatment. There were no apparent behavioral effects after injection of either of the antagonist drugs. Animals continued to drink, eat, and groom like controls.
Antibodies
Two anti-green fluorescent protein (GFP) antibodies, both of which crossreact with and identify EYFP, were used, one a rabbit polyclonal antibody and the other a mouse monoclonal antibody (1 : 1000; Abcam, Cambridge, MA, USA). Anti-rabbit GFAP (1 : 1000) was raised against purified bovine GFAP as an immunogen and used to mark astrocytes. Rabbit Anti-NG2 chondroitin sulfate proteoglycan polyclonal antibody (1 : 250; Millipore, Bedford, MA, USA) was raised against immunoaffinity purified NG2 chondroitin sulfate proteoglycan from rat. MBP (1 : 500; Sternberger and Sternberger, Baltimore, MD, USA) as a protein marker for myelin and O1 (1 : 500; Millipore) as a lipid marker for oligodendroglial cells were used. Rabbit polyclonal Olig2 antibody (1 : 500; Abcam) was generated with a synthetic peptide from the N-terminal region of human Olig2, conjugated to an immunogenic carrier protein. Two different mouse monoclonal anti-calbindins were used: anti-calbindin-D-28K (clone CB-955, ascites fluid, IgG1 isotype, raised against bovine kidney calbindin, 1 : 1000; Sigma), and calbindin D-28K raised against chicken that specifically stains the 45 Ca-binding spot (McAb 300, lot 18(F), 1 : 1000; Swant, Bellinzona, Switzerland). Both antibodies yielded Purkinje cell-specific staining identical to that reported previously.26, 28 Anti-parvalbumin (ascites fluid, 1 : 1000; Sigma), a monoclonal antibody secreted by the PARV-19 hybridoma cell line, was produced by immunization with purified frog muscle parvalbumin. Rabbit polyclonal anti-PDGFRα antibody (1 : 350, Abcam) was generated by immunization of synthetic peptide derived from internal sequence of human PDGFRα. Mouse anti-neuronal nuclei (NeuN) monoclonal antibody (1 : 500; Millipore) was generated by immunization of purified cell nuclei from mouse brain, and was used to detect postmitotic neurons.
Immunohistochemistry
Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with 0.9% NaCl in 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The brains were then removed from the skull and post-fixed in 4% paraformaldehyde at 4 °C for 48 h. The cerebella were cryoprotected through a series of buffered sucrose solutions: 10% (2 h), 20% (2 h), and 30% (overnight), and then embedded in OCT and frozen for cryosectioning. Transverse sections were cut on a cryostat at 40 μm thickness through the extent of the cerebellum and collected as free-floating sections for immunohistochemistry.
Cerebellar sections for fluorescent immunohistochemistry were processed as described previously.50 Briefly, tissue sections were washed thoroughly blocked with 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and then incubated in 0.1 M PBS containing 0.1% Triton-X and the primary antibody for 16–18 h at 4 °C. Sections were then rinsed and incubated for 2 h at room temperature in a mixture of Alexa 546-conjugated goat anti-rabbit IgG, Alexa 488-conjugated goat anti-mouse IgG, and Alexa 643-conjugated goat anti-mouse pig IgG (Molecular Probes Inc., Eugene, OR, USA) in a 1 : 2000 dilution. After several rinses in 0.1 M PBS, sections were cover-slipped in non-fluorescing mounting medium (Fluorsave Reagent, Calbiochem, La Jolla, CA, USA). Photomicrographs were captured with a SPOT Cooled Color digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA), mounted on a Zeiss microscope (Thornwood, NY, USA), and assembled using Adobe Photoshop (version 9; San Francisco, CA, USA) without manipulation.
Statistical analysis
Data are presented as mean±S.E.M. Each experimental group had at least six mice. All immunohistochemical analyses were performed in triplicate. Analysis of variance (ANOVA) with Tukey’s post hoc tests were used to compare differences between groups for all quantitative analyses. Statistical significance was accepted at P≤0.05.
Abbreviations
- BG:
-
Bergmann glia
- CaBP:
-
calbindin binding protein
- E:
-
embryonic day
- EYFP:
-
extended yellow fluorescent protein
- GFAP:
-
glial fibrillary acidic protein
- GFP:
-
green fluorescent protein
- MBP:
-
myelin basic protein
- NMDA:
-
N-methyl-D-aspartate
- NG2:
-
nerve/glial antigen2
- OCE/R:
-
Olig2-Cre-ERT2/Rosa26-EYFP
- OPC:
-
oligodendrocyte precursor cell
- P:
-
postnatal day
- PBS:
-
phosphate buffered saline
- PCE/R:
-
Plp-Cre-ERT2/Rosa26-EYFP
- PDGFRα:
-
platelet-derived growth factor-α receptor
- Plp:
-
proteolipid
- PVL:
-
periventricular leukomalacia
- t.i.:
-
tamoxifen injection
- UCL:
-
unilateral carotid ligation
References
Levine JM, Reynolds R, Fawcett JW . The oligodendrocyte precursor cell in health and disease. Trends Neurosci 2001; 24: 39–47.
Stallcup WB . The NG2 proteoglycan: past insights and future prospects. J Neurocytol 2002; 31: 423–435.
Raff MC, Miller RH, Noble M . A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 1983; 303: 390–396.
Kondo T, Raff M . Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 2000; 289: 1754–1757.
Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol 2003; 161: 169–186.
Aguirre A, Gallo V . Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci 2004; 24: 10530–10541.
Aguirre AA, Chittajallu R, Belachew S, Gallo V . NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol 2004; 165: 575–589.
Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 2008; 11: 1392–1401.
Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L et al. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci 2010; 30: 12036–12049.
Michalski JP, Anderson C, Beauvais A, De Repentigny Y, Kothary R . The proteolipid protein promoter drives expression outside of the oligodendrocyte lineage during embryonic and early postnatal development. PLoS One 2011; 6: e19772.
Zhu X, Bergles DE, Nishiyama A . NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008a; 135: 145–157.
Zhu X, Hill RA, Nishiyama A . NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol 2008b; 4: 19–26.
Guo F, Ma J, McCauley E, Bannerman P, Pleasure D . Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci 2009; 29: 7256–7270.
Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE . NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010; 68: 668–681.
Doerflinger NH, Macklin WB, Popko B . Inducible site-specific recombination in myelinating cells. Genesis 2003; 35: 63–72.
Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J et al. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 2000; 25: 317–329.
Zhou Q, Wang S, Anderson DJ . Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 2000; 25: 331–343.
Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y . The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol 2002; 12: 1157–1163.
Liu W, Shen Y, Plane JM, Pleasure DE, Deng W . Neuroprotective potential of erythropoietin and its derivative carbamylated erythropoietin in periventricular leukomalacia. Exp Neurol 2011; 30: 227–239.
Shen Y, Liu XB, Pleasure DE, Deng W . Axon-glia synapses are highly vulnerable to white matter injury in the developing brain. J Neurosci Res 2012; 90: 105–121.
Mallon BS, Shick HE, Kidd GJ, Macklin WB . Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci 2002; 22: 876–885.
Hirrlinger PG, Scheller A, Braun C, Quintela-Schneider M, Fuss B, Hirrlinger J et al. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol Cell Neurosci 2005; 30: 291–303.
Le Bras B, Chatzopoulou E, Heydon K, Martínez S, Ikenaka K, Prestoz L et al. Oligodendrocyte development in the embryonic brain: the contribution of the plp lineage. Int J Dev Biol 2005; 49: 209–220.
Baracskay KL, Kidd GJ, Miller RH, Trapp BD . NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia 2007; 55: 1001–1010.
Yamada K, Watanabe M . Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int 2002; 77: 94–108.
Ozol K, Hayden JM, Oberdick J, Hawkes R . Transverse zones in the vermis of the mouse cerebellum. J Comp Neurol 1999; 412: 95–111.
Chung SH, Marzban H, Croci L, Consalez GG, Hawkes R . Purkinje cell subtype specification in the cerebellar cortex: Ebf2 acts to repress the zebrin II-positive Purkinje cell phenotype. Neuroscience 2008; 153: 721–732.
Chung SH, Calafiore M, Plane JM, Pleasure DE, Deng W . Apoptosis inducing factor deficiency causes reduced Mitofusion 1 expression and patterned Purkinje cell degeneration. Neurobiol Dis 2011; 41: 445–457.
Muller T, Moller T, Berger T, Schnitzer J, Kettenmann H . Calcium entry through kainate receptors and resulting potassium channel blockade in Bergmann glial cells. Science 1992; 256: 1563–1566.
Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D et al. Localization of neuronal and glial glutamate transporters. Neuron 1994; 13: 713–725.
Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC et al. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia 2008; 56: 200–208.
Sellers DL, Maris DO, Horner PJ . Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci 2009; 29: 6722–6733.
Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010; 6: 578–590.
Deng W, Pleasure J, Pleasure D . Progress in periventricular leukomalacia. Arch Neurol 2008; 65: 1291–1295.
Deng W . Neurobiology of injury to the developing brain. Nat Rev Neurol 2010; 6: 328–336.
Volpe JJ . Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009; 8: 110–124.
Rivest S . Molecular insights on the cerebral innate immune system. Brain Behav Immun 2003; 17 pp 13–19.
Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ . Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol 2006; 497: 199–208.
Richardson WD, Young KM, Tripathi RB, McKenzie I . NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 2011; 70: 661–673.
Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H . Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci 2007; 25: 3489–3498.
Komitova M, Zhu X, Serwanski DR, Nishiyama A . NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol 2009; 512: 702–716.
Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M . Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 2008; 28: 10434–10442.
Yuasa S, Kawamura K, Kuwano R, Ono K . Neuron-glia interrelations during migration of Purkinje cells in the mouse embryonic cerebellum. Int J Dev Neurosci 1996; 14: 429–438.
Altman J, Bayer SA . Development of the Cerebellar System. Relation to Its Evolution, Structure, and Functions. CRC Press: Boca Raton, 1997: pp121–150.
Yamada K, Fukaya M, Shibata T . Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol 2000; 418: 106–120.
Karadottir R, Cavelier P, Bergersen LH, Attwell D . NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 2005; 438: 1162–1166.
De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M et al. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci 2011; 31: 12650–12662.
Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, Soulika A . Disruption of NMDA receptors in oligodendroglial lineage cells does not alter their susceptibility to experimental autoimmune encephalomyelitis or their normal development. J Neurosci 2012; 32: 639–645.
Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1: 4.
Sillitoe RV, Benson MA, Blake DJ, Hawkes R . Abnormal dysbindin expression in cerebellar mossy fiber synapses in the mdx mouse model of Duchenne muscular dystrophy. J Neurosci 2003; 23: 6576–6585.
Acknowledgements
This work was supported by grants from the NIH (RO1 NS059043 and RO1 ES015988 to WD and RO1 NS025044 to DEP), National Multiple Sclerosis Society (to WD and DEP), Feldstein Medical Foundation (to WD), and Shriners Hospitals for Children (to WD and DEP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Verkhratsky
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Chung, SH., Guo, F., Jiang, P. et al. Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum. Cell Death Dis 4, e546 (2013). https://doi.org/10.1038/cddis.2013.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.74
Keywords
This article is cited by
-
The Role of the Oligodendrocyte Lineage in Acute Brain Trauma
Neurochemical Research (2017)
-
The p38α mitogen-activated protein kinase is a key regulator of myelination and remyelination in the CNS
Cell Death & Disease (2015)