Abstract
Neuroinflammation associated with degenerative central nervous system disease and injury frequently results in oligodendrocyte death. While promoting oligodendrocyte viability is a major therapeutic goal, little is known about protective signaling strategies. We report that in highly purified rat oligodendrocytes, interferon gamma (IFNγ) activates a signaling pathway that protects these cells from tumor necrosis factor alpha (TNFα)-induced cytotoxicity. IFNγ protection requires Jak (Janus kinase) activation, components of the integrated stress response and NF-κB activation. Although NF-κB activation also occurred transiently in the absence of IFNγ and presence of TNFα, this activation was not sufficient to prevent induction of the TNFα-responsive cell death pathway. Genetic inhibition of NF-κB translocation to the nucleus abrogated IFNγ-mediated protection and did not change the cell death induced by TNFα, suggesting that NF-κB activation via IFNγ induces a different set of responses than activation of NF-κB via TNFα. A promising candidate is the NF-κB target cFLIP (cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein), which is protease-deficient caspase homolog that inhibits caspase-3 activation. We show that IFNγ-mediated protection led to upregulation of cFLIP. Overexpression of cFLIP was sufficient for oligodendrocyte protection from TNFα and short hairpin RNA knockdown of cFLIP-abrogated IFNγ -mediated protection. To determine the relevance of our in vitro finding to the more complex in vivo situation, we determined the impact on oligodendrocyte death of regional cFLIP loss of function in a murine model of neuroinflammation. Our data show that downregulation of cFLIP during inflammation leads to death of oligodendrocytes and decrease of myelin in vivo. Taken together, we show that IFNγ-mediated induction of cFLIP expression provides a new mechanism by which this cytokine can protect oligodendrocytes from TNFα-induced cell death.
Similar content being viewed by others
Main
Interferon gamma (IFN-γ), the only type-II class IFN, has a paradoxical role in modulating cell function. It is critical for innate and adaptive immunity, but has multiple other functions. In the central nervous system (CNS), IFNγ has contrasting effects on the oligodendrocyte progenitor cells (O-2A/OPCs) that generate myelin-producing oligodendrocytes. O-2A/OPCs show suppressed division when exposed to IFNγ.1, 2, 3 However, when O-2A/OPCs differentiate into oligodendrocytes, IFNγ becomes pro-apoptotic.4, 5, 6, 7 Although IFNγ has a critical role in the pathogenesis of immune-mediated demyelinating disease;8, 9 the response of committed oligodendrocytes to IFNγ is more complex. For example, tumor necrosis factor alpha (TNFα) can show enhanced cytotoxicity in oligodendrocytes and transformed human neural cell lines when co-exposed with IFNγ.3, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
In contrast with reported toxic effects of IFNγ on oligodendrocytes, other studies did not see negative effects on mature oligodendrocytes5, 9, 20 or saw protection of glial lineage cells. IFNγ protects the Oli-neu oligodendrocyte-like cell line from reactive oxygen and nitrogen species,21 and overexpression of IFNγ before the induction of experimental autoimmune encephalomyelitis (EAE) protected oligodendrocytes from immune-mediated damage.9 The mechanism of such protection remains elusive.
We now report that IFNγ protects purified, committed oligodendrocytes from TNFα-mediated apoptosis via Janus kinase (Jak)-mediated activation of the stress kinase PKR (double-stranded RNA-dependent protein kinase) and NF-κB-induced expression of cFLIP (cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein), which inhibits caspase activation. Moreover, gain-of-function and loss-of-function experiments show that cFLIP is necessary and sufficient for oligodendrocyte protection from TNFα. These results demonstrate induction of cFLIP in a stress response and NF-κB-dependent manner, leading to inhibition of caspase-mediated apoptosis, and reveal an important role for cFLIP in oligodendrocyte protection in vivo.
Results
IFNγ protects galactocerebroside-positive oligodendrocytes from TNFα-mediated toxicity and caspase activation
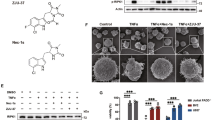
Contrary to previous findings on O-2A/OPCs demonstrating IFNγ enhances toxicity to TNFα,3 we found that IFNγ protects committed oligodendrocytes (defined by galactocerebroside (GalC) expression22, 23, 24, 25) from TNFα (Figure 1). In cultures of >99.5% GalC+ oligodendrocytes, TNFα exposure caused concentration-dependent cell death within 72 h. Significant cell death was observed with 0.1 ng/ml TNFα (Figure 1a; 44±1.6% to 77±1.8% cell death at 0.1–100 ng/ml, respectively). Co-exposure of oligodendrocytes to IFNγ prevented the TNFα-induced death in a dose-dependent manner (Figure 1b; 77±3.3% cell death with 20 ng/ml TNFα versus 24±9% and 10±7.8% with 20 ng/ml TNFα and 1 or 10 ng/ml IFNγ, respectively).
IFNγ protects GalC+ oligodendrocytes from TNFα-induced cell death and inhibits caspase activation. (a) Oligodendrocyte differentiation was induced with thyroid hormone for 5–7 days and treated with TNFα (0.1, 1, 10 and 100 ng/ml) for 72 h. Live oligodendrocytes were MTT+ and GalC+, and had DAPI+ nuclei. Data are plotted as mean±S.E.M. of at least three independent experiments. ***P<0.0001, relative to control treatment; ANOVA followed by Bonferroni’s post hoc test. (b) Oligodendrocyte viability was determined after 72 h in the presence of 20 ng/ml TNFα and increasing concentrations of IFNγ (0.1, 1, 10 and 100 ng/ml). **P<0.001, relative to control treatment; ANOVA followed by Bonferroni’s post hoc test. (c) Oligodendrocytes were treated with IFNγ and/or with TNFα for 72 h and immunostained with anti-activated caspase-3 antibody, anti-GalC and DAPI. Note the pyknotic nucleus in the TNFα condition. Images were optimized for brightness and contrast. Scale bars, 25 μm. (d) Oligodendrocytes treated as in b were quantified and expressed as % activated caspase-3+/GalC+ cells. Data are plotted as mean±S.E.M. of at least three independent experiments. **P<0.001 relative to control treatment; ANOVA followed by Bonferroni’s post hoc test. (e) IFNγ promoted oligodendrocyte protection from TNFα while control conditioned media (Ctl-CM), IFNγ-treated oligodendrocyte CM (IFNγ-CM) and IFNγ blocking antibodies plus IFNγ failed to protect oligodendrocytes. Data are plotted as mean±S.E.M. of at least three independent experiments. ***P<0.0001, **P<0.001 relative to control treatment; ANOVA followed by Bonferroni’s post hoc test. NS, no significant difference
TNFα exposure also induced caspase-3 activation in oligodendrocytes, as indicated by caspase-3-positive pyknotic nuclei (Figure 1c), which was prevented by IFNγ (Figures 1c and d). Activated caspase-3 was detected in 24.5±5% or 25.8±5% of oligodendrocytes with control or IFNγ treatment, respectively. TNFα exposure significantly increased the proportion of caspase-3-positive oligodendrocytes to 70.3±6%. IFNγ- plus TNFα-treated cells were not significantly different from controls (35.8±4%; Figure 1d), thereby suggesting that IFNγ signaling inhibits caspase activation.
IFNγ -mediated protection also represented a direct effect on oligodendrocytes. Conditioned medium (CM) collected for 3 days from oligodendrocytes exposed to IFNγ (IFNγ-CM), or control oligodendrocyte growth media (Ctl-CM), did not protect oligodendrocytes from TNFα (Figure 1e), thereby suggesting that no secreted or secondary factor is responsible for protection. In contrast, IFNγ neutralizing antibodies inhibited oligodendrocyte survival in IFNγ/TNFα co-treatments (Figure 1e).
Jak activation is required for IFNγ-mediated oligodendrocyte protection
We next asked whether IFNγ-mediated protection relied on canonical signaling pathways. Canonical IFNγ signaling involves ligand binding to the type-2 IFN receptor, activation of Jak and subsequent recruitment, activation, and dimerization of the signal transducer and activators of transcription proteins (STATs).26, 27 Jak/STAT signaling in oligodendrocytes typically involves Jak2-mediated activation of STAT1 and to a lesser extent STAT3, which translocates to the nucleus and activates transcription.
Oligodendrocytes treated with 10 ng/ml IFNγ alone showed increased STAT1 phosphorylation within minutes, lasting for at least 3 h without changing total STAT1 (Figure 2a). Phosphorylated STAT3 was undetectable (data not shown) and levels of total STAT3 did not change at the time points investigated (Figure 2a). Exposure to TNFα alone did not induce STAT1 phosphorylation, but when cells were co-treated with IFNγ plus TNFα, we observed greater levels of phospho-STAT1 than in cells exposed to IFNγ alone (Figure 2a).
IFNγ activates Jak/STAT signaling in oligodendrocytes. (a) Whole-cell lysates were obtained from oligodendrocytes treated as indicated and probed by western blotting for pSTAT1 (Y701), STAT1 and STAT3, normalized to β-tubulin. pSTAT1 (Y701) levels were represented as pSTAT1 (Y701)/STAT1. (b–d) Oligodendrocytes were treated as indicated, and RNA was isolated for RT-qPCR of IRF-1, SOCS1, SOCS3 and GAPDH. Data are plotted as mean±S.E.M. of at least three independent experiments. ***P<0.0001, **P<0.001 compared with control treatment; ANOVA followed by Bonferroni’s post hoc test
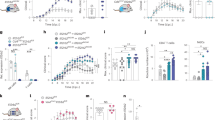
STAT1 activation of transcription in oligodendrocytes was examined by analysis of expression of IFN response factor 1 (IRF-1). These studies indicated robust induction of STAT1-mediated transcription (Figure 2b). Exposure to IFNγ induced a >100-fold increase in IRF-1 gene expression 5 days after treatment. Activation of this pathway was maintained despite significant upregulation of suppressor of cytokine signaling (SOCS) transcripts. SOCS1 was transcriptionally upregulated over 50-fold for up to 5 days (Figure 2c), whereas SOCS3 was upregulated 5-fold at 1 day but returned to control levels by 5 days (Figure 2d).
We further found that IFNγ-mediated protection required Jak activation. Treatment of oligodendrocytes (72 h) with the Jak inhibitor AG-490 showed no significant cytotoxicity at 1 μM (Figure 3a) versus DMSO vehicle control and abolished IFNγ-mediated phosphorylation of STAT1 (Figure 3b). Jak inhibition by AG-490 also abolished IFNγ-mediated protection (Figure 3c), suggesting that Jak activation is required for protection.
Jak activation is required for oligodendrocyte protection. (a) Dose–response curve of Jak inhibitor AG-490 (0.1, 1, 5, 10 and 100 μM) on oligodendrocytes after 3 days in culture. Data are plotted as mean±S.E.M. of at least three independent experiments. ***P<0.0001 relative to DMSO vehicle control; ANOVA followed by Bonferroni’s post hoc test. (b) AG-490 (1 μM) effectively inhibited STAT1 phosphorylation. Whole-cell lysates were obtained from oligodendrocytes treated with AG-490 or DMSO vehicle control and probed with antibodies against phospho-STAT1 (Y701) and β-tubulin. (c) Oligodendrocytes were treated with IFNγ and/or TNFα in the presence of AG-490 (1 μM) or DMSO vehicle control for 72 h. Data are plotted as mean±S.E.M. of technical triplicates from a representative experiment repeated three times. ***P<0.0001, **P<0.01 relative to DMSO vehicle control; ANOVA followed by Bonferroni’s post hoc test. (d) Oligodendrocytes were infected with lentivirus (pLKO.1) expressing either scrambled shRNA or STAT1-targeting shRNA for 4 h, allowed 48 h for recovery and treated with IFNγ (1, 10 and 100 ng/ml) and 20 ng/ml TNFα as indicated for 72 h. Data are plotted as mean±S.E.M. of at least three independent experiments and were compared with scrambled shRNA control. (e) Oligodendrocytes were infected with lentivirus (pLKO.1) expressing either scrambled shRNA or STAT1-targeting shRNA for 4 h, allowed 48 h for recovery and whole-cell lysates were collected for western blotting to determine the degree of STAT1 knockdown. Lysates were probed with antibodies against STAT1 and β-tubulin. Lentiviral shRNA constructs targeting STAT1 protein specifically reduced STAT1 expression by >90% compared with scrambled control shRNA. (f) Oligodendrocytes were infected with lentivirus expressing scrambled shRNA as in e and treated as indicated. Data are plotted as mean±S.E.M. of at least three independent experiments. *P<0.05 compared with control treatment by ANOVA followed by Bonferroni’s post hoc test. NS, not significant
While Jak activation was necessary for protection of cells from TNFα, STAT1 protein was necessary for TNFα to act as a cell death signal (consistent with previous studies in a macrophage cell line where non-phosphorylated STAT1 interacts with the TNF receptor (TNFR1) leading to caspase activity and subsequent apoptosis28). Analysis of cell death after 3d showed that knockdown of STAT1 by short hairpin RNA (shRNA) itself prevented TNFα-induced oligodendrocyte death, as compared to scrambled control-treated cells (Figures 3d and f).
PKR is required for oligodendrocyte protection and activates the integrated stress response
It was previously reported that IFNγ protects oligodendrocytes from immune-mediated apoptosis by activating the unfolded protein response (UPR).9 The UPR is one component of the integrated stress response (ISR) that enables cells to react to stressors through phosphorylation of the eukaryotic translation initiation factor-α (eIF2α), while at the same time upregulating specific protective mRNAs.29 To examine the role of the ISR in IFNγ-mediated protection, we first determined whether protection depended on PKR. PKR is a well-characterized IFNγ target and is critical for IFN-mediated antiviral response.30, 31
Oligodendrocytes exposed to IFNγ (10 ng/ml) with or without TNFα (20 ng/ml) for 72 h showed elevated PKR levels (Figure 4a). We also found robust activation of eIF2α (which is phosphorylated by PKR), while total eIF2α levels were unchanged (Figure 4a). As further evidence of ISR activation, we found significant increases (3.2 to >5-fold) in mRNA levels of Chop (also known as GADD153; Figure 4b).
PKR activation is required for IFNγ–mediated oligodendrocyte protection. (a) Whole-cell lysates were obtained from oligodendrocytes treated as indicated for 72 h and probed by western blotting for PKR, phospho-eIF2α, eIF2α, phospho-PERK, PERK and β-tubulin. (b) Oligodendrocytes were treated as indicated, and RNA was isolated for RT-qPCR of Chop and GAPDH. Data are plotted as mean±S.E.M. of at least three independent experiments. *P<0.05, **P<0.01 compared with control treatment; ANOVA followed by Bonferroni’s post hoc test. (c) Oligodendrocytes were infected with lentivirus expressing either scrambled shRNA or (pGIPZ) PKR-targeting shRNA for 4 h, allowed 48 h for recovery and whole-cell lysates were collected for western blotting to determine the degree of PKR knockdown. Lysates were probed with antibodies against PKR and β-tubulin. Lentiviral shRNA constructs targeting PKR protein specifically reduced STAT1 expression by >80% compared with scrambled control shRNA. (d) Oligodendrocytes were infected with lentivirus expressing scrambled shRNA (see also Figure 3d for full set of scrambled shRNA controls) or (pGIPZ) PKR-targeting shRNA for 4 h, allowed 48 h for recovery and treated with IFNγ and/or TNFα. ***P<0.0001 compared with scrambled shRNA-infected, control-treated oligodendrocytes; ANOVA followed by Bonferroni’s post hoc test. (e) RNA was isolated as in b and levels of BiP mRNA were normalized to that of GAPDH
Oligodendrocytes transduced with lentiviral shRNA constructs targeting PKR (reduced PKR expression 80% compared with scr-shRNA controls; Figure 4c) showed marked suppression of IFNγ protection from TNFα (Figure 4d). Cell viability in PKR knockdown cells exposed to TNFα with IFNγ did not differ from cells exposed to TNFα alone (Figure 4d). In contrast, cells expressing scrambled shRNA showed no change in IFNγ-mediated protection (Figure 3f).
In contrast with effects of IFNγ on PKR activation, we found no activation of PKR-like endoplasmic reticulum (ER) kinase (PERK), which was recently implicated in IFNγ-mediated protection of oligodendrocytes9, 32, 33 (Figure 4a). Furthermore, we found no change in expression of the ER chaperone protein BiP (Figure 4e), which is associated with accumulation of mis- or unfolded proteins in the ER. These data demonstrate that PKR is required for IFNγ-mediated protection from TNFα, and IFNγ protection involves activation of the ISR, without BiP or PERK activation.
NF-κB activation is required for IFNγ-mediated protection
Having established PKR as a critical regulator of IFNγ-mediated protection, we next investigated the possible mechanism of its actions. NF-κB, a PKR target,34, 35 can act as a general cytoprotective factor through activation of manganese superoxide dismutase and Bcl-2.36 NF-κB complexes are expressed throughout the CNS, activating diverse genes in a cell-specific manner,37 but the contribution of oligodendrocytic NF-κB activation to protection remains elusive (see for review Blank and Prinz38).
We next asked whether activation of NF-κB is critical for IFNγ-mediated protection. Exposure of oligodendrocytes to IFNγ, TNFα or the combination significantly enhanced NF-κB reporter activity after 3 h with the greatest increase in TNFα and TNFα plus IFNγ treatments. NF-κB activation returned to baseline levels by 24 h, with no significant difference in cells exposed to control medium, TNFα, IFNγ or TNFα plus IFNγ (Figure 5a). The absence of prolonged NF-κB activation in IFNγ and TNFα plus IFNγ exposed cultures was surprising, considering that strong activation of PKR was still present at 72 h. Therefore, we analyzed IκB phosphorylation and degradation, which represents a critical step in NF-κB activation by facilitating NF-κB translocation to the nucleus.34, 39, 40 Whole-cell lysates from oligodendrocytes, treated for 24 h, showed no significant changes in levels of IκB-α or pIκB(S32/36) in response to IFNγ (Figure 5b). Although levels of pIκB were lower and the ratio of pIκB-α to IκB dropped from 0.8 and 0.7 in the IFNγ- and TNFα-treated cells, respectively, to 0.5 in the combination treatment, the decrease was not reflected in any changes in NF-κB reporter activation.
IFNγ-mediated protection requires activation of NF-κB. (a) Oligodendrocytes were infected with lentivirus containing NF-κB luciferase reporter constructs for 16 h and allowed 48 h for recovery. Treatment was as indicated for 3, 24 or 48 h. Data are plotted as mean±S.E.M. of at least three independent experiments. *P<0.05, compared with control treatment; ANOVA followed by Bonferroni’s post hoc test. (b) Whole-cell lysates were obtained from oligodendrocytes treated for 24 h as indicated and probed by western blotting for pIκB-α (S32/36), IκB-α and β-actin. Protein expression was quantified and normalized to actin intensity. (c) Oligodendrocytes were infected with lentivirus (pCDH) containing either no insert (empty vector; pCDH-e.v.) or cDNA encoding FLAG-tagged, non-phosphorylatable IκB (pCDH-FLAG-IκB(SR) for 16 h, allowed 48 h for recovery and whole-cell lysates were collected to determine the expression of transgene. Lysates were probed with antibodies against FLAG and β-tubulin. (d) Oligodendrocytes were infected with lentivirus (pCDH) as in c and treated as indicated. Data are plotted as mean±S.E.M. of at least three independent experiments. **P<0.0001 compared with e.v.-infected, control-treated oligodendrocytes; ANOVA followed by Bonferroni’s post hoc test
Prolonged activation of NF-κB seems not to have a role in the IFNγ protection, but transcription of NF-κB targets might act in the IFNγ rescue from TNFα even if NF-κB itself is no longer active. To test whether NF-κB activation was necessary for the IFNγ rescue, we infected oligodendrocytes with lentivirus overexpressing a FLAG-tagged, non-phosphorylatable IκB-α mutant (IκB-super repressor (IκB-SR); Figure 5c), which functions as a super repressor of NF-κB activation and nuclear translocation.41 Expression of IκB-SR blocked IFNγ-mediated protection from TNFα (Figure 5d), suggesting that activation of NF-κB-responsive genes is necessary.
Oligodendrocyte protection requires NF-κB-mediated upregulation of cFLIP
Having found that NF-κB is critical to IFNγ-mediated protection, and that such protection prevents caspase activation, we next sought to identify the specific effector(s) that prevent this step in activation of cell death. In these studies, we focused on the long form of flice inhibitory protein cFLIPL (referred here as cFLIP), a protease-deficient caspase-8 homolog.42 Although there is no previously reported linkage of IFNγ to cFLIP, cFLIP is upregulated by NF-κB activity43 and directly inhibits activation of caspase-8,44 a critical effector in TNFα-mediated death signaling.
Western blot and mRNA analysis revealed that cFLIP transcript and protein levels were significantly (~50%) upregulated by IFNγ alone or in combination with TNFα (compared with TNFα alone; Figures 6a and b). Although TNFα alone increased the active fragment/form of caspase-8, the addition of IFNγ reduced the amount of active fragment/form of caspase-8 by over 50% (Figure 6b). As caspase-3 activity is regulated by the protease activity of active fragment/form of caspase-8, this would explain our earlier observations that IFNγ significantly reduced the proportion of TNFα-induced caspase-3-positive oligodendrocytes (Figures 1c and d).
IFNγ significantly increases cFLIPL in a Jak-dependent manner, which is both sufficient and necessary for protection from TNFα. (a) Oligodendrocytes were treated as indicated and RNA was isolated for RT-qPCR of cFLIP and GAPDH. Data are plotted as mean±S.E.M. of at least three independent experiments. *P<0.05, **P<0.01 compared with control treatment; ANOVA followed by Bonferroni’s post hoc test. (b) Whole-cell lysates were obtained from oligodendrocytes treated for 72 h as indicated and probed by western blotting for cFLIP, activated caspase-8 and β-tubulin. Protein expression was quantified and normalized to tubulin intensity. (c) Oligodendrocytes were infected with control (RFP) or cFLIP overexpressing lentivirus (pCDH) constructs for 16 h, given 48 h to recover and treated with TNFα for 72 h. Cells were then fixed and stained with anti-GalC and cFLIP antibodies. Images show representative cell cultures treated with the indicated conditions. Images were optimized for brightness and contrast. Note the pyknotic nucleus in the control virus, TNFα condition (c′). (d) Oligodendrocytes were infected with lentivirus (pLKO.1) expressing either scrambled shRNA or cFLIP-targeting shRNA for 16 h, allowed 48 h for recovery and whole-cell lysates were collected to determine the degree of cFLIP knockdown. Lysates were probed with antibodies against cFLIP and β-tubulin. Lentiviral shRNA constructs targeting cFLIP protein specifically reduced cFLIP expression by >50% compared with scrambled control shRNA. (e) Oligodendrocytes were infected with lentivirus (pLKO.1) expressing either scrambled shRNA or cFLIP-targeting shRNA for 16 h, allowed 48 h for recovery and treated for 72 h with IFNγ and/or TNFα. (A full set of scr ShRNA control vectors is shown in Figure 3f). Data are plotted as mean±S.E.M. of at least three independent experiments. *P<0.05, **P<0.01 compared with scrambled shRNA-infected, control-treated oligodendrocytes; ANOVA followed by Bonferroni’s post hoc test. (f) Oligodendrocytes were pre-treated with 5 μm AG-490 or DMSO for 16 h, then co-treated with DMSO, 5 μm AG-490, IFNγ and/or TNFα as indicated for 6 h. RNA was isolated for RT-qPCR of cFLIP and GAPDH. Data are plotted as mean±S.E.M. of three independent experiments. *P<0.05, ***P<0.001; ANOVA followed by Bonferroni’s post hoc test
To confirm that expression of cFLIP protected oligodendrocytes from TNFα-induced death, we infected oligodendrocytes with lentivirus expressing full-length cFLIP (cFLIP OE) or control virus (Ctl) and exposed cells to TNFα. In control-infected oligodendrocytes ( cells), we found that 72 h exposure to TNFα led to significant cell death (Figure 6c, lower panel), with loss of GalC+ membranes and fragmented apoptotic nuclei (Figure 6c′). In contrast, oligodendrocytes infected with cFLIP expressing virus (Figure 6c, upper panel) revealed high levels of cFLIP expression, membrane integrity and viability after TNFα exposure.
We also found that cFLIP expression was necessary for IFNγ-mediated protection. Targeting of cFLIP using lentiviral shRNA decreased protein expression by ~50%, as compared with scrambled shRNA-treated oligodendrocytes (Figure 6d). Quantification of viable MTT+/GalC+ cells revealed that IFNγ was unable to confer oligodendrocyte protection from TNFα in cFLIP knockdown conditions. In contrast to the nearly complete protection provided by IFNγ in parental cells (Figure 1b), survival of oligodendrocytes was decreased by ~75% with TNFα plus cFLIP shRNA, and decreased by ~60% in cFLIP knockdown cells exposed to IFNγ plus TNFα (Figure 6e).
Having shown that IFNγ-mediated protection requires Jak activation (Figure 2), we next asked whether the inhibition of Jak/STAT signaling impaired the IFNγ-induced upregulation of cFLIP. As shown in Figure 6f, treatment of oligodendrocytes with the Jak inhibitor AG-490 abolished IFNγ-mediated upregulation of cFLIP expression.
Loss of cFLIP during inflammation increases oligodendrocyte death in vivo
To address whether cFLIP expression is important for oligodendrocyte viability in vivo under inflammatory conditions, we performed loss-of-function experiments in a mouse model of sustained inflammation using IL-1βXAT mice. The IL-1βXAT transgene consists of the human IL-1β transcript driven by the GFAP promoter separated by a lox-STOP-lox cassette. Exposure to Cre recombinase induces IL-1β expression, reaching peak inflammation at 14 days.45, 46, 47 We further confirmed that intracranial injection of Cre recombinase-expressing lentivirus caused upregulation of a number of inflammatory markers, including IFNγ and TNFα (Figure 7d).
cFLIP knockdown during sustained neuroinflammation in vivo increases oligodendrocyte death and decreases myelin staining intensity. (a and b) Adult IL-1βXAT mice were injected with transgene activating Cre-expressing lentivirus and either scrambled shRNA (Cre-virus scr-shRNA) or cFLIP-targeting (Cre-virus cFLIP) shRNA and aged for 2 weeks. Brain sections were stained with GSTπ antibodies, DAPI and for TUNEL. (b′) Higher magnification views of TUNEL+/GSTπ+ oligodendrocytes. (c) Quantification of TUNEL+/GSTπ+ oligodendrocytes per field. Data are plotted as mean±S.D. of five fields per section from two mice. *P<0.05, t-test. (d) Adult IL-1βXAT mice were injected with Cre-expressing virus or control virus and RNA was isolated from brain for RT-PCR of TNFα, IFNγ and cFLIP. Note that the upregulation of cFLIP levels during IL-1β-mediated inflammation coincides with the absence of oligodendrocytes death in this mouse model. (e) Sections from adult IL-1βXAT mice injected with the Cre-expressing virus into the corpus callosum of both hemispheres, with one side also receiving a cFLIP-specific shRNA-expressing virus (right, r) and the contralateral side receiving a non-targeting, scrambled shRNA-expressing virus (left, l) that were stained with FluoroMyelin. (f) Quantification of fluorescence intensity in arbitrary units from three sections per mouse and two mice. *P<0.05 t-test
IL-1βXAT mice show no overt oligodendrocyte death despite significant leukocyte infiltration into the CNS,45, 46, 47 suggesting the existence of a protective mechanism that shields the oligodendrocytes in this mouse model from inflammation-induced apoptotic cell death. As we also found that in the inflamed CNS of IL-1βXAT mice there is an upregulation of cFLIP expression (Figure 7d), we next determined whether increased c-FLIP expression following induction of inflammation might be involved in protecting oligodendrocytes from the effects of inflammation an in vivo model.
To determine whether the high levels of cFLIP occurring in vivo were necessary for preventing oligodendrocyte death, we injected both hemispheres of IL-1βXAT transgenic mice with the Cre-expressing virus into the corpus callosum, with one side also receiving a cFLIP-specific shRNA-expressing virus (Figure 7b) and the contralateral side receiving a non-targeting, scrambled shRNA-expressing virus (Figure 7a). Controls also consisted of intracranial injections as above into non-transgenic (WT) littermates. To confirm that the induction of inflammation is specific to the site of transgene activation, we labeled sections with CD45 and found, consistent with previous reports,45 robust leukocyte infiltration into the CNS of transgenic mice 2 weeks after transgene activation, while WT brains lacked detectable CD45 staining (data not shown).
Introduction of cFLIP shRNA into the CNS was associated with increased oligodendrocyte death as indicated by increases in the number of TUNEL+ and GSTπ+ cells in the corpus callosum (Figures 7b and 7b′). In contrast, inflammation alone (and injection of scrambled control virus) did not result in significant oligodendrocyte death in the corpus callosum as determined by the paucity of TUNEL+/GSTπ+ cells (Figure 7a). Furthermore, when we assessed gross myelination of the corpus callosum with FluoroMyelin staining, we found a significant decrease in myelin staining intensity in the hemisphere that received cFLIP shRNA compared with the scrambled shRNA-treated hemisphere within the same brain section (Figures 7e and f).
Discussion
We have identified a novel mechanism by which IFNγ protects oligodendrocytes from TNFα-mediated apoptosis. In this pathway, IFNγ exposure activates Jak, leading to phosphorylation of STAT1, PKR and NF-κB activation, and ultimately to increased expression of c-FLIPL, which inhibits caspase activation and TNFα-induced cell death. We further show that cFLIPL, a target of NF-κB, is both necessary and sufficient to protect oligodendrocytes from TNFα-induced cell death.
Our results suggest a number of novel and unexpected interactions between IFNγ and TNFα. Activation of ISR, including PERK was reported to be critical in providing protection,48 although the molecular mechanisms leading to inhibition of cell death were not clear. Unlike previous reports9, 32, 33, 48 suggesting that protection by IFNγ requires activation of the ISR, we did not observe phosphorylation of PERK and BiP expression induced by IFNγ. This suggests that the UPR was not activated in our system, and that there was no ER stress induced by IFNγ or TNFα. The significant upregulation in oligodendrocytes of PKR was essential in conferring IFNγ protection, which represents a novel finding.
Induction of cFLIP by IFNγ signaling might, however, not be the sole event that prevents TNFα-induced cell death. Wesemann et al.49 showed in macrophages that IFNγ induced depletion of Stat1 through phosphorylation, thus limiting the ability of cytoplasmic STAT1 to associate with TNFR1 and initiate cell death, while at the same time enhancing NF-κB activation that could act as a pro-survival signal. While we saw increased STAT1 phosphorylation by IFNγ, and STAT1 protein was necessary for TNFα to cause oligodendrocyte death, we did not see enhanced NF-κB activation in the presence of IFNγ. Our results instead showed that TNFα transiently activates NF-κB to a higher degree than IFNγ alone or in combination, and that NF-κB activation has ceased at the time we see rescue from cell death. It hence seems unlikely that IFNγ protects oligodendrocytes through increased NF-κB activation. On the other hand, we found that eliminating the ability of cells to activate NF-κB also prevented IFNγ rescue.
If NF-κB activation is necessary for protection, why is it then that NF-κB activation by TNFα leads to cell death? A possible and attractive explanation is provided by the work by Ganster et al.50 who showed that the relative orientation of juxtaposed NF-κB-Stat (SIE) cis-elements determines the ability of TNFα and IFNγ to induce gene transcription. Interestingly, their data suggest that IFNγ-induced activation involved STAT DNA binding while TNFα’s induction of NF-κB DNA binding was unaffected by the presence or absence of STAT1. In other words, it seems likely that IFNγ-induced STAT1 phosphorylation, which leads to nuclear translocation, results in a NF-κB–STAT complex that activates a different set of genes than activation of NF-κB and TNFα in the absence of nuclear STAT1. In support of such a differential activation is a preliminary analysis of the rat c-Flip (=Cflar) promoter region (15 kb upstream to 5 kb downstream of transcription start site), using the Transfac database and Patch 1.0 software (BIOBASE Biological Databases, Beverly, MA, USA), revealing two NFκB binding sites at position −3943 and −4405 bp, and several STAT1/STAT3 binding sites (position −2539, −4397 and −4452 bp). Differential expression could be highly cell type specific, consistent with different outcomes of NF-κB activation in astrocytes, microglia or O-2A/OPCs (see for review Blank and Prinz38).
A limitation of our study is that our experiments using a JAK inhibitor did not allow us to clearly define the role of phosphorylated STAT1, as JAK inhibition would also lead to inhibition of PKR,51 which we show is critical for IFNγ rescue. We can also not exclude the involvement of other, perhaps parallel acting, pathways that might contribute to IFNγ–mediated protection. We detected significant and sustained Chop activation in the absence of cell death, but the relevance of this activation is not clear. CHOP expression is generally considered protective in oligodendrocytes, but in Schwann cells (the myelinating cells of the peripheral nervous system), Chop activation is pro-apoptotic.52, 53
Irrespective of the relative contribution of various IFNγ-induced pathways, we were able to identify cFLIP as a key effector protein in protection. cFLIP was both necessary for IFNγ-mediated protection and sufficient to confer protection on its own against TNFα-mediated killing. cFLIP inhibits caspase-8 activation, thereby decreasing caspase-3 activation, providing direct means of regulating executioner pathways in cell death. Despite the well-described role of cFLIP as an anti-apoptotic factor,54 its protective role has only been described in the context of activation of the terminal complement cascade and Fas-mediated apoptosis.44 Based on our studies, cFLIP appears to also offer an instance of a protein naturally induced by a relevant inflammatory cytokine that confers protection.
Our mechanistic and cell-specific studies used a highly purified cell culture system that offered the advantage of enabling cell-specific analyses but has the disadvantage of not resembling complex inflammatory in vivo environments. Evidence that cFLIP expression might be relevant in vivo, however, came from our studies on IL-1βXAT mouse. This model was uniquely well suited for our studies, as IL-1β expression leads to robust leukocyte recruitment to the CNS, astrocyte activation and induction of pro-inflammatory cytokines but no oligodendrocyte toxicity.45, 47 Although the reasons for the absence of toxicity were initially not clear, our analyses showed a robust upregulation of cFLIP expression in IL-1βXAT mice, thereby raising the question of whether this might be protective. Indeed, cFLIP knockdown with shRNA led to a significant increase in TUNEL+ oligodendroyctes in addition to a decrease in global myelin staining intensity. While the number of TUNEL+ oligodendrocytes seems small in relation to the decrease in FluoroMyelin staining intensity, this may be owing to the rapid pace at which apoptotic oligodendrocytes are cleared from the white matter, which may be accelerated during neuroinflammation and macrophage infiltration.45
The possibility of IFNγ-induced cFLIP upregulation acting as a protective pathway seems consistent with studies, reporting that elimination of IFNγ expression increases EAE severity,55, 56 and that its expression can protect against disease onset,57 oligodendrocyte death and demyelination induced by cuprizone.58 However, the protective effect might depend on the concentration, timing and/or source of IFNγ, as there are also studies showing that administration of IFNγ to multiple sclerosis patients, or in animals with EAE, can worsen inflammation and clinical symptoms.59, 60 Although the upregulation of IFNγ in the model used here is defined by the time of viral induction, we have not yet identified the source of IFNγ in our model. We have described significant infiltration of peripheral macrophages, neutrophils and T cells.45 In addition, preliminary data show that induction of inflammation also induced proliferation of microglia. All these cells are potential candidates to be activated and to respond with a variety of factors that are released including IFNγ. It is thus likely that the context in which IFNγ acts on oligodendrocytes has a role in the activation of pro-survival or pro-apoptotic signaling cascades.
As the ability of IFNγ to worsen CNS damage in certain situations makes it hard to conceive of utilizing this cytokine in protective strategies, it is important to identify molecular mechanisms involved in conferring its protective effects. The finding that cFLIP loss of function inhibits IFNγ-mediated protection in vitro and increases oligodendrocyte apoptosis in vivo provides proof of concept that cFLIP is a good candidate protein to promote oligodendrocyte viability. These findings may provide a means of defining the context in which the protective mechanisms induced by IFNγ can be induced.
Materials and Methods
Cell culture
All animal procedures were reviewed and approved by the University Committee on Animal Resources of the University of Rochester Medical Center. Optic nerves were isolated from (Thermo Fisher Scientific, Waltham, MA, USA) postnatal day 7 mixed sex rat pups and processed as described previously.2 Briefly, nerves were minced, digested with papain and collagenase, and were triturated. Cells were enriched by magnetic cell sorting (Miltenyi Biotec, San Diego, CA, USA) using magnetic bead-conjugated A2B5 antibody and grown in defined media, DMEM/F12 containing insulin, transferring and Sato components61 as previously described (Tanner et al.2). Cells were expanded in the presence of PDGF-AA (10 mg/ml; PeproTech, Rocky Hill, NJ, USA) on poly-l-lysine–coated Nunc tissue culture plastic (Thermo Fisher Scientific) for only one passage before plating for differentiation. Cells were never exposed to serum. Differentiation of O-2A/OPCs into oligodendrocytes involved decreasing the PDGF-AA concentration to 1 ng/ml and adding thyroid hormone (40 nM, consisting of 30 ng/ml thyroxine and 36 ng/ml triiodothrionine (Sigma-Aldrich, St. Louis, MO, USA) for 5–7 days. At this point, all cells were GalC+. We did not use CNTF to induce maturation as this cytokine also actives the Jak/STAT pathway, thus potentially confounding our finding. We define these postmitotic, premyelinating and/or myelinating cells as ‘committed oligodendrocytes’. Similar results were obtained from corpus callosum-derived O-2A/OPCs, but differentiation into oligodendrocytes was more uniform in optic nerve-derived cells. Oligodendrocyte cultures were treated with 10 ng/ml recombinant rat IFNγ (Peprotech) and 20 ng/ml rat TNFα (Peprotech) unless otherwise noted. Oligodendrocytes were treated with AG-490 (Enzo Life Sciences, Farmingdale, NY, USA) as described. Cell viability was scored as % Ctl MTT+/GalC+ cells.
Immunocytochemistry
Cells were plated as indicated and processed as described previously (Tanner et al.2). Cell staining was performed with the following antibodies diluted in HBSS (Invitrogen, Carlsbad, CA, USA) with 5% fetal calf serum, 0.5% sodium azide and 0.1% Triton X-100: anti-GalC (mouse IgG3, hybridoma supernatant; 1 : 10 dilution), activated caspase-3 (Rb IgG; 1 : 500; Abcam, Cambridge, UK), anti- cFLIPL (Rb IgG; 1 : 50; MBL International, Woburn, MA, USA). Secondary antibodies were Alexa-conjugated 488 or 568 (1 : 2000; Invitrogen). Nuclei were stained with DAPI (Invitrogen). MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue; Sigma-Aldrich) was diluted in PBS (5 mg/ml), sterile filtered and applied to oligodendrocytes, which were treated as indicated, for 4 h immediately before fixation. Soluble MTT was converted to an insoluble precipitate marking cells as having active mitochondrial dehydrogenases, and therefore, alive. Under a variety of culture conditions, dead oligodendrocytes can appear to be viable with elaborate GalC+ membranes, despite being MTT− and lacking DAPI+ nuclei. For this reason, we considered viable oligodendrocytes to be only GalC+/DAPI+/MTT+. Oligodendrocytes were grown on Nunc tissue culture grade plastic (Thermo Fisher Scientific), were imaged on an inverted fluorescence Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) and images were acquired with a Spot camera using Spot software (Diagnostic Instruments, Sterling Heights, MI, USA) as described previously (Tanner et al.2).
Immunoblotting
O-2A/OPCs were plated on 10-cm Nunc tissue culture dishes (Thermo Fisher Scientific) at 105 cells per dish. Cells were induced to differentiate as described above and treated as indicated. Whole-cell lysates were obtained in modified RIPA buffer containing protease and phosphatase inhibitors, and were subjected to SDS-PAGE, transferred to PVDF and probed with primary antibodies against the following substrates: STAT1 (Rb IgG; 1 : 1000) phospho-STAT1 (Tyr701; Rb IgG; 1 : 1000), STAT3 (Rb mAb clone 79D7; Rb IgG; 1 : 2000), phospho-STAT3 (Ser727; Rb IgG; 1 : 1000), eIF2α (Rb IgG; 1 : 1000), phospho-eIF2α (Ser51; Rb mAB clone 119A11; Rb IgG, 1 : 1000) and IκBα (Rb IgG; 1 : 1000), all obtained from Cell Signaling Technology (Danvers, MA, USA); PKR (Ms IgG2a; 1 : 1000), PERK (H300; Rb IgG; 1 : 1000), phospho-PERK (Thr981; Rb IgG; 1 : 1000), β-tubulin (H-235; Rb IgG; 1 : 2000) and β-actin-HRP (C4; Ms IgG1; 1 : 20 000), all obtained from Santa Cruz Biotechnology (Dallas, TX, USA); anti-active caspase-8 (Rb IgG; 1 : 500) and anti-cFLIPL (58 kDa; Rb IgG; 1 : 100), all obtained from MBL International and anti-FLAG (M2; Ms IgG1; 1 : 2000) obtained from Sigma-Aldrich. Secondary antibodies were all HRP conjugates (1 : 2500; Santa Cruz Biotechnology).
PCR
Rat oligodendrocytes were treated as indicated, and RNA was isolated from cells and converted to cDNA following standard protocols. Quantitative real-time PCR primers and probes for IRF-1, Chop, BiP and Caspase 12 have been previously published and were synthesized by IDT.5, 62 Taqman assays (Thermo Fisher Scientific) were used for SOCS1, SOCS3, cFLIP and GAPDH. Samples were run multiplexed with GAPDH, and expression levels were calculated using the ΔΔCT method.
NF-κB reporter assay
Oligodendrocytes were infected with lentivirus containing NF-κB luciferase reporter as indicated for lentivirus propagation. After 3, 24 or 48 h treatment with indicated cytokines or control media, oligodendrocytes were treated with a Renilla Luciferase Assay Kit (Biotium, Hayward, CA, USA) according to directions. Luciferase buffer and oligodendrocyte lysate were combined in Corning Costar white opaque microplates (Corning, Tewksbury, MA, USA) and luciferase was determined in relative units on plate reader. Relative luciferase intensity was normalized to the protein content of each well.
Lentivirus propagation
Constructs containing shRNA sequences for STAT1, cFLIP and scrambled shRNA control sequence were purchased from Open Biosystems (now GE Healthcare, Lafayette, CO, USA) as inserts into the pLKO.1 vector. shRNA against PKR was an insert in the pGIPZ vector (GE Healthcare). Propagation was performed essentially as previously described (Tanner et al.2). Viral supernatant was sterile filtered and added to oligodendrocytes in culture for 4–16 h, as indicated, and cells were recovered for 48 h. Infection efficiency was routinely tested for shRNA backbones expressing fluorescence constructs and was consistently >90%. Expression constructs for IκB(SR) (kind gift from Guzman and Jordan;41 32,36 S→A), cFLIP (GE Healthcare, clone ID 7376876) and nls-Cre (plasmid 12106; Addgene, Cambridge, MA, USA)63 were cloned into pCDH-CMV-MCS (CD500B-1; GE Healthcare). An NF-κB promoter response element sequence was conjugated to a renilla luciferase gene and then cloned into FG12 (Addgene). Virus was prepared as described previously (Tanner et al.2). Lentivirus was prepared for stereotactic injection by concentration with PEG 6000 as previously described.64 Essentially, viral supernatant was sterile filtered and precipitated in PEG 6000 (final concentration 8.5%) and NaCl (final concentration 0.3 M) in 100 mM phosphate buffer (PB). After 1.5 h at 4 °C, samples were concentrated by centrifugation at 7,000 × g for 10 min. Pellets were resuspended in 50 mM Tris-HCl, pH 7.4. pCDH-nlsCRE was tested for recombinase activity in 293 T cells that were selected for pCALNL-DsRed expression (plasmid 13769; Addgene).65
Stereotactic injection
Adult IL-1βXAT mice were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and secured in a stereotactic frame (David Kopf Instruments, Tujunga, CA, USA). Two burr holes were drilled at 0.26 mm anterior and 1.0 mm lateral of the bregma, one in each hemisphere. A 10-μl syringe with a 33-G needle was inserted to a depth of 1.5 mm (corpus callosum) deep over the course of 3 min. In each animal, the right hemisphere received a total of 4 μl containing concentrated CRE-expressing lentivirus and cFLIP-shRNA-expressing lentivirus. The left hemisphere received a total of 4 μl containing concentrated CRE-expressing lentivirus and scrambled shRNA-expressing lentivirus. After each injection, the needle was left in place for 5 min and removed over the course of 1 min. Scalps were sutured and mice were given analgesic and monitored for 6 h continuously and subsequently at least once per day for 12 days. All mice, two IL-1βXAT mice and three WT control littermates, survived surgery and only transgenic mice demonstrated CD45+ cell infiltration into the CNS after CRE injection.
Tissue staining
Adult IL-1βXAT mice were deeply anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and transcardially perfused with 10 ml ice-cold PBS with heparin (2 U/ml) followed by fresh 0.4% PFA. Brains were removed from skulls and postfixed in 0.4% PFA overnight at 4 °C. PFA was replaced by cryoprotectant (30% sucrose in PBS) for at least 48 h until brains sunk at 4 °C. Brains were mounted in cryoprotectant surrounded by dry ice for sectioning on a Leica SM 2000R sliding knife apparatus (Leica Microsystems, Buffalo Grove, IL, USA). Sections were cut at 30 μm and stored at 4 °C. Sections were rinsed four times each in 100 mM PB, pH 7.4) and blocked in vehicle consisting of 10% normal goat serum and 0.3% Triton X-100 in 100 mM PB. Anti-CD45 (Ms IgG1; 1 : 1000, AbD Serotec, Raleigh, NC, USA) primary antibody was diluted in vehicle. Secondary antibody was Alexa-conjugated 568 (1 : 2000; Invitrogen). Nuclei were stained with DAPI (Invitrogen). Free-floating brain sections were assayed for myelin staining intensity using FluoroMyelin (1 : 300 in 100 mM PB, pH 7.4; Invitrogen). Tissue sections were mounted onto glass slides in Fluoromount-G (Southern Biotech, Birmingham, AL, USA) and were analyzed on an upright fluorescence Nikon eclipse 80i microscope (Nikon) and pictures were taken with a Cool SNAP EZ (Photometrics, Tuscon, AZ, USA) camera using NIS Elements software (Nikon). TUNEL staining was performed using the ApopTag Fluorescein Direct apoptosis detection kit (Chemicon, now EMD Millipore, Billerica, MA, USA) according to manufacturer’s protocol combined with anti-GSTπ (Ms IgG1; 1 : 1000; BD Biosciences, San Jose, CA, USA) staining. Secondary antibodies for GSTπ was Alexa-conjugated 568 (1 : 2000; Invitrogen). Nuclei were stained with DAPI (Invitrogen). Tissue sections were mounted onto glass slides in Fluoromount-G (Southern Biotech) and were analyzed as a series of 1 μm z-stacks with a Leica SP2 confocal microscope with a × 40 oil objective (Leica Microsystems).
RT-PCR
Conventional RT-PCR was performed on RNA isolated from adult IL-1βXAT mouse brains using the following primers: TNF-α, forward 5′-GACAAGGCTGCCCCGACTA-3′ and reverse 5′-TTTCTCCTGGTATGAGATAGCAAATC-3′; IFNγ, forward 5′-TACTGCCACGGCACAGTCATTGAA-3′ and reverse 5′-TGGACCTGTGGGTTGTTGACCTCAACCTCAAACTTGGC-3′; cFLIP, forward 5′-GGCGGCCATTCTCATCTTCTCGG-3′ and reverse 5′-ACCTCGGCAGACACAGGGCT-3′; and GAPDH, forward 5′-GGTGAAGGTCGGTGTGAACG-3′ and reverse 5′-CTCGCTCCTGGAAGATGGTG-3′.
Statistical analyses
All experiments were performed at least three independent times and bar on graphs represent mean±S.E.M. unless otherwise indicated. Statistical tests used were the Student’s t-test and one-way ANOVA with Bonferroni’s multiple comparison post hoc test as indicated in the figure legends. P-values <0.05 were considered statistically significant and are marked by ‘*’. Data that did not reach statistical significance are marked by ‘NS’ (no significant difference). All statistical analyses were performed using Prism4.0 (GraphPad software, Graphpad, La Jolla, CA, USA).
Abbreviations
- IFN-γ:
-
interferon gamma
- TNFα:
-
tumor necrosis factor alpha
- Jak:
-
Janus kinase
- ISR:
-
integrated stress response
- cFLIP:
-
cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein
- CNS:
-
central nervous system
- O-2A/OPC:
-
oligodendrocyte progenitor cells
- EAE:
-
experimental autoimmune encephalomyelitis
- PKR:
-
double-stranded RNA-dependent protein kinase R
- GalC:
-
galactocerebroside
- CM:
-
conditioned medium
- STATs:
-
signal transducer and activators of transcription proteins
- IRF-1:
-
IFN response factor 1
- SOCS:
-
suppressor of cytokine signaling
- UPR:
-
unfolded protein response
- eIF2α:
-
eukaryotic translation initiation factor-α
- PERK:
-
PKR-like endoplasmic reticulum kinase
- IκB-SR:
-
IκB-super repressor.
References
Agresti C, D'Urso D, Levi G . Reversible inhibitory effects of interferon-gamma and tumour necrosis factor-alpha on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci 1996; 8: 1106–1116.
Tanner DC, Cherry JD, Mayer-Proschel M . Oligodendrocyte progenitors reversibly exit the cell cycle and give rise to astrocytes in response to interferon-gamma. J Neurosci 2011; 31: 6235–6246.
Andrews T, Zhang P, Bhat NR . TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res 1998; 54: 574–583.
Baerwald KD, Popko B . Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J Neurosci Res 1998; 52: 230–239.
Lin W, Harding HP, Ron D, Popko B . Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J Cell Biol 2005; 169: 603–612.
Chew LJ, King WC, Kennedy A, Gallo V . Interferon-gamma inhibits cell cycle exit in differentiating oligodendrocyte progenitor cells. Glia 2005; 52: 127–143.
Vartanian T, Li Y, Zhao M, Stefansson K . Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med 1995; 1: 732–743.
Lees JR, Cross AH . A little stress is good: IFN-gamma, demyelination, and multiple sclerosis. J Clin Invest 2007; 117: 297–299.
Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest 2007; 117: 448–456.
Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G et al. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol 1998; 153: 801–813.
Jurewicz A, Matysiak M, Tybor K, Kilianek L, Raine CS, Selmaj K . Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain 2005; 128: 2675–2688.
Mayer M, Noble M . N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci USA 1994; 91: 7496–7500.
Merrill JE, Benveniste EN . Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 1996; 19: 331–338.
Selmaj K, Papierz W, Glabinski A, Kohno T . Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol 1995; 56: 135–141.
Pouly S, Becher B, Blain M, Antel JP . Interferon-gamma modulates human oligodendrocyte susceptibility to Fas-mediated apoptosis. J Neuropathol Exp Neurol 2000; 59: 280–286.
Buntinx M, Gielen E, Van Hummelen P, Raus J, Ameloot M, Steels P et al. Cytokine-induced cell death in human oligodendroglial cell lines. II: Alterations in gene expression induced by interferon-gamma and tumor necrosis factor-alpha. J Neurosci Res 2004; 76: 846–861.
Scurlock B, Dawson G . Differential responses of oligodendrocytes to tumor necrosis factor and other pro-apoptotic agents: role of ceramide in apoptosis. J Neurosci Res 1999; 55: 514–522.
Choi C, Jeong E, Benveniste EN . Caspase-1 mediates Fas-induced apoptosis and is up-regulated by interferon-gamma in human astrocytoma cells. J Neurooncol 2004; 67: 167–176.
Fransen L, Van der Heyden J, Ruysschaert R, Fiers W . Recombinant tumor necrosis factor: its effect and its synergism with interferon-γ on a variety of normal and transformed human cell lines. Eur J Cancer Clin Oncol 1986; 22: 419–426.
Balabanov R, Strand K, Goswami R, McMahon E, Begolka W, Miller SD et al. Interferon- -oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci 2007; 27: 2013–2024.
Lin Y, Jamison S, Lin W . Interferon-gamma activates nuclear factor-kappa B in oligodendrocytes through a process mediated by the unfolded protein response. PLoS One 2012; 7: e36408.
Ahlgren SC, Wallace H, Bishop J, Neophytou C, Raff MC . Effects of thyroid hormone on embryonic oligodendrocyte precursor cell development in vivo and in vitro. Mol Cell Neurosci 1997; 9: 420–432.
Durand B, Gao FB, Raff M . Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J 1997; 16: 306–317.
Gard AL, Pfeiffer SE . Glial cell mitogens bFGF and PDGF differentially regulate development of O4+GalC- oligodendrocyte progenitors. Dev Biol 1993; 159: 618–630.
Warrington AE, Barbarese E, Pfeiffer SE . Stage specific, (O4+GalC-) isolated oligodendrocyte progenitors produce MBP+ myelin in vivo. Dev Neurosci 1992; 14: 93–97.
Darnell JE Jr ., Kerr IM, Stark GR . Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994; 264: 1415–1421.
Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD . How cells respond to interferons. Annu Rev Biochem 1998; 67: 227–264.
Wesemann DR, Benveniste EN . STAT-1 alpha and IFN-gamma as modulators of TNF-alpha signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J Immunol 2003; 171: 5313–5319.
Klann E, Dever TE . Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci 2004; 5: 931–942.
Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 2007; 6: 975–990.
Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C et al. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 2006; 70: 1032–1060.
Lin W, Kunkler PE, Harding HP, Ron D, Kraig RP, Popko B . Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-. Am J Pathol 2008; 173: 1508–1517.
Lin W, Popko B . Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci 2009; 12: 379–385.
Kumar A, Haque J, Lacoste J, Hiscott J, Williams BR . Double-stranded RNA-dependent protein kinase activates transcription factor NF-kappa B by phosphorylating I kappa B. Proc Natl Acad Sci USA 1994; 91: 6288–6292.
Kumar A, Yang YL, Flati V, Der S, Kadereit S, Deb A et al. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-kappaB. EMBO J 1997; 16: 406–416.
Mattson MP, Camandola S . NF-κB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 2001; 107: 247–254.
O'Neill LA, Kaltschmidt C, NF- kappa B . a crucial transcription factor for glial and neuronal cell function. Trends Neurosci 1997; 20: 252–258.
Blank T, Prinz M . NF-κB signaling regulates myelination in the CNS. Front Mol Neurosci 2014; 7: 47.
Deb A, Haque SJ, Mogensen T, Silverman RH, Williams BR . RNA-dependent protein kinase PKR is required for activation of NF-kappa B by IFN-gamma in a STAT1-independent pathway. J Immunol 2001; 166: 6170–6180.
Karin M, Ben-Neriah Y . Phosphorylation meets ubiquitination:the control of NF-kB activity. Annu Rev Immunol 2000; 18: 621–663.
Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci USA 2002; 99: 16220–16225.
Budd RC, Yeh WC, Tschopp J . cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol 2006; 6: 196–204.
de Wilt Lham, Kroon J, Jansen G, de Jong S, Peters GJ, Kruyt FAE . Bortezomib and TRAIL: A perfect match for apoptotic elimination of tumour cells? Crit Rev Oncol/Hematol 2012; 85: 363–372.
Cudrici C, Niculescu F, Jensen T, Zafranskaia E, Fosbrink M, Rus V et al. C5b-9 terminal complex protects oligodendrocytes from apoptotic cell death by inhibiting caspase-8 processing and up-regulating FLIP. J Immunol 2006; 176: 3173–3180.
Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK . Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci 2007; 27: 9301–9309.
Shaftel SS, Griffin WS, O'Banion MK . The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation 2008; 5: 7.
Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK . Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest 2007; 117: 1595–1604.
Lin W, Lin Y, Li J, Fenstermaker AG, Way SW, Clayton B et al. Oligodendrocyte-specific activation of PERK signaling protects mice against experimental autoimmune encephalomyelitis. J Neurosci 2013; 33: 5980–5991.
Wesemann DR, Qin H, Kokorina N, Benveniste EN . TRADD interacts with STAT1-alpha and influences interferon-gamma signaling. Nat Immunol 2004; 5: 199–207.
Ganster RW, Guo Z, Shao L, Geller DA . Differential effects of TNF-alpha and IFN-gamma on gene transcription mediated by NF-kappaB-Stat1 interactions. J Interferon Cytokine Res 2005; 25: 707–719.
Bleiblo F, Michael P, Brabant D, Ramana CV, Tai T, Saleh M et al. JAK kinases are required for the bacterial RNA and poly I:C induced tyrosine phosphorylation of PKR. Int J Clin Exp Med 2013; 6: 16–25.
D'Antonio M, Feltri ML, Wrabetz L . Myelin under stress. J Neurosci Res 2009; 87: 3241–3249.
Southwood CM, Garbern J, Jiang W, Gow A . The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron 2002; 36: 585–596.
Micheau O . Cellular FLICE-inhibitory protein: an attractive therapeutic target? Expert Opin Ther Targets 2003; 7: 559–573.
Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol 1996; 156: 5–7.
Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA . IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol 1996; 157: 3223–3227.
Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC et al. Intrathecal delivery of IFN-gamma protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol 2001; 167: 1821–1829.
Gao X, Gillig TA, Ye P, D'Ercole AJ, Matsushima GK, Popko B . Interferon-gamma protects against cuprizone-induced demyelination. Mol Cell Neurosci 2000; 16: 338–349.
Panitch HS, Hirsch RL, Schindler J, Johnson KP . Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology 1987; 37: 1097–1102.
Renno T, Taupin V, Bourbonniere L, Verge G, Tran E, De Simone R et al. Interferon-gamma in progression to chronic demyelination and neurological deficit following acute EAE. Mol Cell Neurosci 1998; 12: 376–389.
Bottenstein JE, Sato GH . Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA 1979; 76: 514–517.
Wang Y, Ren Z, Tao D, Tilwalli S, Goswami R, Balabanov R . STAT1/IRF-1 signaling pathway mediates the injurious effect of interferon-gamma on oligodendrocyte progenitor cells. Glia 2010; 58: 195–208.
Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM . Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci USA 2001; 98: 11450–11455.
Kutner RH, Zhang XY, Reiser J . Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc 2009; 4: 495–505.
Matsuda T, Cepko CL . Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci USA 2007; 104: 1027–1032.
Acknowledgements
This work was supported by a New York State Department of Health Award (grant no. CO23691–MMP). DCT was supported in part by a Kirchstein NRSA fellowship (NINDS; T32NS051152) and a postdoctoral fellowship from the NMSS (FG1812A1). The authors are grateful for insight and comments from Drs. Fred Strathmann, Ibro Ambeskovic, Brett Stevens and Christoph Proschel, and thank Jonathan D Cherry, Brendan Carlin and Addie Bardin for their expert technical assistance.
Author Contributions
DCT designed and conducted experiments, formulated the underlying hypothesis and wrote the manuscript draft. AC designed and conducted experiments, and contributed to the writing of the manuscript. MM-P conducted experiments, provided resources, contributed to the design, analysis and interpretation of data. KMO’B provided the IL-1βXAT mice, and contributed to the design and interpretation of the in vivo data. MN provided resources, and was involved in the writing and editing of the manuscript, and providing critically intellectual content.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by L Greene
Rights and permissions
About this article
Cite this article
Tanner, D., Campbell, A., O'Banion, K. et al. cFLIP is critical for oligodendrocyte protection from inflammation. Cell Death Differ 22, 1489–1501 (2015). https://doi.org/10.1038/cdd.2014.237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.237
This article is cited by
-
Effects of anti-depressant treatments on FADD and p-FADD protein in rat brain cortex: enhanced anti-apoptotic p-FADD/FADD ratio after chronic desipramine and fluoxetine administration
Psychopharmacology (2016)
-
The interplay between inflammation and metabolism in rheumatoid arthritis
Cell Death & Disease (2015)