Abstract
BOK/MTD was discovered as a protein that binds to the anti-apoptotic Bcl-2 family member MCL-1 and shares extensive amino-acid sequence similarity to BAX and BAK, which are essential for the effector phase of apoptosis. Therefore, and on the basis of its reported expression pattern, BOK is thought to function in a BAX/BAK-like pro-apoptotic manner in female reproductive tissues. In order to determine the function of BOK, we examined its expression in diverse tissues and investigated the consequences of its loss in Bok−/− mice. We confirmed that Bok mRNA is prominently expressed in the ovaries and uterus, but also observed that it is present at readily detectable levels in several other tissues such as the brain and myeloid cells. Bok−/− mice were produced at the expected Mendelian ratio, appeared outwardly normal and proved fertile. Histological examination revealed that major organs in Bok−/− mice displayed no morphological aberrations. Although several human cancers have somatically acquired copy number loss of the Bok gene and BOK is expressed in B lymphoid cells, we found that its deficiency did not accelerate lymphoma development in Eμ-Myc transgenic mice. Collectively, these results indicate that Bok may have a role that largely overlaps with that of other members of the Bcl-2 family, or may have a function restricted to specific stress stimuli and/or tissues.
Similar content being viewed by others
Main
The BCL-2 family of proteins are major regulators of apoptosis, which according to structure and function can be divided into three groups.1 One group (including BCL-2, BCL-XL, BCL-W, MCL-1 and A1) inhibits apoptosis, whereas the other two groups – namely the BAX/BAK-like subfamily2 and the BH3-only proteins (including BIM, NOXA, PUMA, BIK, HRK, BAD and BID)3 promote apoptosis. Complex interactions between the pro-survival and the two sub-groups of pro-apoptotic members determine whether a cell lives or dies. The BH3-only proteins are essential for initiation of apoptosis signalling and are activated in a cell death stimulus as well as cell type specific manner.1, 3, 4 BAX and BAK on the other hand are essential for mitochondrial outer membrane permeabilisation (MOMP), which unleashes the caspase cascade for cellular demolition.1 They appear to be activated by direct binding by certain BH3-only proteins,2, 4 as well as (indirectly) by liberation from pro-survival BCL-2-like proteins.5, 6
BAX and BAK have overlapping actions in the ‘mitochondrial’ apoptotic pathway.7 Mice lacking either BAK or BAX alone are largely normal (with the exception of sterility of Bax−/− males), but Bax/Bak doubly deficient mice show remarkable developmental abnormalities, including persistent inter-digital webbing and brain malformations that are the likely cause of the early post-natal death of most of these animals.7 Fibroblasts and lymphocytes from Bax−/−Bak−/− mice are profoundly resistant to a broad range of apoptotic stimuli, such as cytokine deprivation, DNA damage7, 8 and even enforced expression of BH3-only proteins,9, 10 although, as expected,11 not to ‘death receptor’-induced killing. Intriguingly, a very small number (<10% on a C57BL/6 background) of Bax/Bak doubly deficient mice survive to early adulthood and apart from a remarkable lymphadenopathy,8 many of their organs, such as the kidney, liver, heart and lungs display no overt developmental defects and function normally.7 This may indicate that an additional protein can function in a BAX/BAK-like manner. Alternatively, an apoptotic process independent of the BAX/BAK-dependent ‘Bcl-2 regulated’ pathway or a non-apoptotic process (e.g., necroptosis) may ensure developmentally programmed cell death to sculpt tissues in Bax−/−Bak−/− mice.
BOK (also known as MTD) was discovered as a protein that bound to pro-survival MCL-1 by screening of a rat ovarian fusion cDNA library in the yeast 2-hybrid system.12 Mouse BOK (mBOK) shows most extensive amino-acid sequence homology to mBAK (87%) and mBAX (73%), with particularly strong conservation across the Bcl-2 Homology (BH) domains and the trans-membrane region.12 Consequently, it is widely assumed that BOK exerts a BAX/BAK-like pro-apoptotic function. In support of this hypothesis, it has been reported that over-expression of BOK can induce apoptosis in certain mammalian cell lines and primary neurons from the dorsal root ganglia of neonatal rats.12, 13, 14 Moreover, in placental samples from pregnant women suffering from pre-eclampsia, Bok expression was found to be associated with abnormally elevated trophoblast cell death.15, 16 Interestingly, somatically acquired loss of copy number of the Bok gene has been detected in diverse human cancers,17 indicating that BOK may function as a tumour suppressor.
To determine the function of Bok, we have examined its expression in diverse tissues and cell types and generated Bok knockout (Bok−/−) mice. Our results show that Bok is expressed most prominently in reproductive tissues and the brain, but is also present at lower levels in most other tissues. However, Bok-deficient mice were of normal appearance, fertile, displayed normal tissue architecture and their lymphoid and myeloid cells were normally sensitive to a range of apoptotic stimuli. These findings may indicate that Bok exerts a function that is largely overlapping with those of BAX and BAK, and may feature most prominently in reproductive organs and the brain, the tissues in which it is most highly expressed.
Results
Bok mRNA is expressed in a wide range of tissues
Earlier studies reported that Bok shows restricted expression in reproductive tissues.12 Consistent with these findings, qPCR analysis demonstrated that Bok mRNA expression was most abundant in the uterus and ovaries of healthy, adult (14-week old) C57BL/6 female mice, but relatively high levels were also seen in the brain (∼1.8-fold less compared with the uterus) followed by the spleen, lung, stomach, small intestine and kidney (Figure 1a). Lower, but still readily detectable levels of Bok mRNA were found in the liver, thymus and lymph nodes, with the weakest expression observed in the heart and bone marrow (BM) (Figure 1a). Western blotting using polyclonal rabbit antibodies raised against a mBOK-derived polypeptide revealed that BOK protein could be readily detected in tissues that expressed high levels of Bok mRNA (Figure 1b).
Bok is expressed in a broad range of tissues and hematopoietic cell types and its levels are not affected by loss of Bax and/or Bak. (a) The levels of Bok mRNA in different tissues of adult (12–16-week old) C57BL/6 (wt) mice are expressed in relation to a fixed reference sample (wt brain). (b) Western blot analysis of major organs from C57BL/6 (wt) mice for BOK expression. Brain extracts from Bok−/− animals were used as a negative control. Probing with an antibody to GAPDH was used as a loading control. (c) Bok mRNA expression in FACS-sorted hematopoietic cell populations from the LN, thymus (thy), and BM in comparison with the reference sample (wt brain; white bar). (d) BOK protein expression in the indicated leukocyte populations was determined by western blot analysis. Cells isolated from the uterus of wt and Bok−/− mice were used as a positive and negative control, respectively, and probing of the membranes with an anti-GAPDH antibody served as a loading control
qPCR analysis of FACS sorted leucocyte sub-populations revealed readily detectable, albeit relatively low, levels of Bok mRNA in T-cell subsets (CD4+CD8−, CD4−CD8+, CD4−CD8− and CD4+CD8+) from the thymus, B and T cells from lymph nodes, and ∼five-fold higher expression in Ter119+ erythroid cells, macrophages and neutrophils from the BM of (wt) C57BL/6 mice (Figure 1c). Western blot analysis revealed the presence of BOK protein in splenic CD8+ T cells, thymocytes (Figure 1d) and bone-marrow derived granulocytes and macrophages (data not shown). Higher levels of BOK protein were observed in total extracts from thymus, spleen and lymph nodes (LN) compared with isolated lymphocyte sub-populations, indicating that non-hematopoietic cells within these tissues express more BOK than lymphoid cells (Figure 1d).
Given the prevailing view that Bok may exert a function akin to those of BAX and BAK, we explored whether loss of either Bax, Bak or both impacted on the expression of Bok. qPCR analysis for Bok mRNA expression comparing extracts of 13 organs from Bax−/−, Bak−/− and Bax−/−Bak−/− mice with those of wt controls revealed that loss of BAK, BAX or both proteins did not significantly alter Bok mRNA levels (Supplementary Figure 1a), with the possible exception of LN from Bax−/− mice, which displayed slightly higher levels of Bok mRNA (wt=0.125, Bax−/−=0.473, P=0.0349). Western blot analysis showed that most tissues from Bax−/− and Bak−/− mice contained similar levels of BOK protein as those from wt animals, although there may be an increase in BOK in the rectum and ovaries from BAX- and BAK-deficient mice (Supplementary Figure 1b).
Generation of Bok−/− mice
The Bok gene was targeted for deletion by introducing LoxP sites that flank half of exon 1 and exon 2, which contains the ATG start site (Figure 2a). Two independent lines of BOK-deficient mice were produced by crossing Bokfl/+ mice with Cre-deleter+/tg females to achieve excision of the targeted region. Intercrosses of Bok+/− mice generated Bok−/−, Bok+/− and Bok+/+ offspring, which were genotyped before being used for examination (Figure 2b). qPCR analysis using primers located in the region of the Bok gene that was not deleted revealed that no Bok mRNA could be detected in tissues from Bok−/− mice (Figure 2c). Similarly, western blot analysis revealed a ∼23-kDa band corresponding to the predicted molecular weight of BOK only in tissues from wt mice, but not those from Bok−/− animals (Figure 2d). Additional western blot analysis of several tissues revealed that loss of BOK did not cause marked alterations in the levels of BAX or BAK proteins (Supplementary Figure 2). Together, these results demonstrated successful inactivation of the Bok gene and that this did not affect the expression of BAX/BAK.
Targeting of the Bok locus. (a) Schematic diagram depicting the wt Bok allele and the targeting construct. Dashed lines demarcate the targeted locus in the Bok gene. The locations of 5′ and 3′ external probes used for Southern blot analysis are indicated. (b) All mice were genotyped before examination. A successful targeted deletion of Bok yielded a 383-bp PCR product, whereas the wt allele produced a 150-bp band when subjected to gel electrophoresis. (c) Bok mRNA levels in wt and Bok−/− mice were determined by qPCR analysis. Data represent the mean±S.D from three mice of each genotype. (d) The absence of BOK protein in mutant mice was confirmed by western blot analysis of Bok−/− tissue extracts along with corresponding tissues from wt controls. Membranes were incubated with rabbit anti–BOK polyclonal antibodies and subsequently with a monoclonal antibody to GAPDH (loading control)
Bok-deficient mice develop normally and organs in Bok−/− mice appear normal
Intercrosses of Bok+/− mice produced Bok−/−, Bok+/− and Bok+/+ offspring at the expected Mendelian ratio (25 : 51 : 23), and Bok−/− mice appeared outwardly normal and healthy when monitored for up to 18 months of age. Moreover, Bok−/− mice of both genders could produce healthy offspring, indicating that BOK is dispensable for normal embryonic development and female or male fertility.
As Bok mRNA is prominently expressed in female reproductive tissues (Figure 1a),12 we examined serial sections of ovaries from early postnatal (day 11), adult (14 weeks) and aged (7 and 12 months) females. Gross histological analysis revealed that ovaries from Bok−/− females were indistinguishable from those of age-matched wt controls. Folliculogenesis was normal and there was no evidence of dysmorphic structures or pathology (Figure 3a). Quantification of the different follicle populations from serial sections showed no significant differences in follicle numbers between wt and Bok−/− ovaries (Figure 3b). Histological examination of uteri from 14-week-old Bok−/− female mice revealed no obvious abnormalities in the luminal epithelium, stroma, glandular epithelium and myometrial layers (Figure 3c).
Reproductive organs in female Bok−/− mice are of normal appearance. Histological sections from ovaries and uteri of 14–16-week-old Bok−/− and wt mice were stained with haematoxylin plus eosin and examined by microscopic analysis. (a) Ovaries of wt and Bok−/− mice at post-natal day 11 (magnification bar=50 μm) and 12 months of age (magnification bar=200 μm). Images of 14 week- and 7-month-old ovaries were also examined but are not shown. (b) Graphs depict the mean score for each follicle type in the ovary, which are expressed as numbers per mm2. Data represent the mean±S.E.M. of follicle numbers from 3–6 mice of each genotype. (c) Images showing the morphology of the uterus from wt and Bok−/− mice shown at × 5 (inset) and × 20 magnification
Subsequently, we examined whether BOK loss might give rise to phenotypic aberrations in the brain, given that it is expressed relatively highly in this organ (Figure 1) and that loss of its relative, BAX, causes abnormal accumulation of neurons in the facial nuclei.18 Microscopic evaluation of serial sections revealed that brains from Bok−/− animals appeared normal (Figure 4a), and no obvious accumulation of motor neurons in the facial nucleus was observed (Figure 4b). A side-by-side comparison of brain sections suggested that Bok−/− mice may have a slightly (∼5%) larger brain, however, there was no statistically significant difference when brain weights were compared.
Brains in Bok−/− mice are of normal appearance. (a) Serial sections of the brain were obtained and stained with crystal violet. (Top panel) Images include the cerebral cortex (Ctx), corpus callosum (CC), septum (Se), striatum (Str), and lateral ventricle (*). (Bottom panel) Transverse sections of the facial nucleus (highlighted with dotted lines) in wt and Bok−/− animals. (b) The number of facial motor neurons in brains from Bok−/− mice were quantified and compared with age- and sex-matched wt controls. Data represent means±S.E.M. from 3–4 mice of each genotype
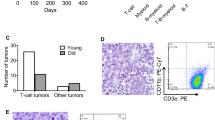
Further histological analysis of eighteen other tissues from 14- to 16-week-old Bok−/− mice, including the kidney, liver, testes, lymph nodes, spleen, thymus and pancreas, did not reveal any obvious abnormalities (Supplementary Figure 3). Intriguingly, Bok−/− females displayed a significant, albeit relatively minor, increase in spleen (wt=68.43±3.123 mg, Bok−/−=85.57±5.618 mg, P=0.0205) and thymus (wt=55.71±2.670 mg, Bok−/−=66.57±1.901 mg, P=0.0062) weights (Figure 5a), but no significant difference was observed in males (Figure 5b). Taken together, these results demonstrate that on its own, BOK is dispensable for normal mouse development and ageing.
Bok−/− female but not male mice have abnormally increased thymus and spleen weight. (a) Spleens and thymi from 9–12-week-old female and (b) male Bok−/− and control wt mice were weighed. Each data point represents an individual mouse (n=7 for each genotype and gender). Statistically significant differences are indicated. *P<0.05, **P<0.01
Lymphoid organs of adult Bok−/− mice have normal hematopoietic cell subset composition
As qPCR and western blot analyses revealed the presence of Bok mRNA and BOK protein, respectively, in lymphoid and particularly myeloid cell subsets (Figure 1), we investigated the impact of Bok deficiency on the development and homeostasis of the hematopoietic compartment by flow cytometric analysis. In particular, we wanted to find out whether the increase in thymus and spleen weights observed in Bok−/− females (Figure 5a) could be attributed to the accumulation of a specific leucocyte sub-population. Total thymic cellularity of Bok−/− mice as well as their content of CD4−CD8− pro-T cells, CD4+CD8+ immature and CD4+CD8− as well as CD4−CD8+ mature thymocytes were comparable to those of wt controls (Figure 6a). Similarly, spleens from Bok−/− mice contained normal numbers of leucocytes overall, immature and mature B cells, CD4+CD8− as well as CD4−CD8+ mature T cells, neutrophils, macrophages and Ter119+ erythroid cells (Figure 6b). Loss of Bok also had no impact on the numbers of pro-B, pre-B, immature and mature B cells, neutrophils, macrophages and nucleated erythroid cells in the BM (Figure 6c). Finally, Bok−/− and wt mice had comparable numbers of lymphocytes, monocytes, neutrophils, eosinophils and basophils in their blood (Figure 6d). These results demonstrate that loss of Bok does not cause defects in the development or homeostasis of hematopoietic cells.
Bok−/− mice have normal hematopoietic cell subset composition. (a) Thymus, (b) spleen and (c) BM were extracted from 9–12-week-old Bok−/− and control wt mice. Single-cell suspensions were prepared, counted, stained with fluorochrome-labelled antibodies against lineage-specific surface markers and analysed by flow cytometry to determine the percentages and (by calculation) numbers of (a) CD4−CD8−, CD4+CD8+, CD4+CD8− and CD4−CD8+ thymocytes, (b) B220+sIgMhisIgDlow as well as B220+sIgMlowsIgDhi mature B cells, CD4+CD8− as well as CD4−CD8+ mature T cells, Mac-1+Gr-1+ granulocytes, Mac-1+Gr-1− macrophages/monocytes and Ter119+ erythroid cells in the spleen and (c) B220+sIg−c-Kit+ pro-B, B220+sIg−c-Kit− pre-B, B220+sIgMhisIgDlow as well as B220+sIgMlowsIgDhi mature B cells, Mac-1+Gr-1+ granulocytes, Mac-1+Gr-1− macrophages/monocytes and Ter119+ nucleated erythroid cells in the bone marrow. (d) Hematopoietic cell subset composition in the blood was determined with an ADVIA automated blood analyser. Results represent mean±S.E.M. of the total numbers of cells of a given subset from 9–12-week-old female mice (n=5 of each genotype). FACS analyses of lymphoid organs from Bok−/− male mice were similarly performed, but these data are not shown
Bok-deficient lymphoid and myeloid cells respond normally to apoptotic stimuli
The absence of pro-apoptotic Bcl-2 family members, such as Bim,19 Puma20 or combined deficiency for Bax and Bak,7, 8 render hematopoietic cells resistant to apoptotic stimuli. As Bok mRNA and protein could be detected in several hematopoietic cell subsets (Figure 1), we investigated whether the loss of Bok could confer a survival advantage upon lymphoid or myeloid cells in the presence of various apoptotic stimuli. FACS-sorted sIg+ B cells, CD4+CD8− as well as CD4−CD8+ mature T cells from lymph nodes, CD4+CD8+ immature thymocytes and bone marrow-derived neutrophils were cultured in the presence of dexamethasone, etoposide, Fas ligand, the BH3 mimetic compound ABT-737 or in simple culture medium (Dulbecco's modified Eagle's medium (DMEM) +10% FCS) lacking cytokines to mimic cytokine deprivation. These cytotoxic insults all rapidly killed the wt hematopoietic cell populations, but loss of BOK failed to confer any protection from these stressors (Figure 7). These results show that on its own, BOK is dispensable for apoptosis induced by a diverse range of death stimuli, at least in lymphoid and myeloid cells.
Lymphoid and myeloid cells from Bok−/− mice are normally sensitive to a broad range of apoptotic stimuli. FACS sorting was used to isolate mature (a) (sIg+) B cells as well as mature (b) CD4+CD8− and (c) CD4−CD8+ T cells from LN (d) CD4+CD8+ (double positive, DP) immature thymocytes from the thymus and (e) Mac-1+Gr-1+ granulocytes from the BM of 9–14-week-old Bok−/− and control wt mice. These cells were exposed in tissue culture to a range of apoptotic stimuli, including the glucocorticoid dexamethasone (1 μM), etoposide (1 μM), crosslinked FasL (100 ng/ml), the BH3 mimetic ABT-737 (240 nM) and growth factor deprivation (DMEM +10% FCS without addition of cytokines). Cell survival was monitored at the indicated times by staining with PI plus FITC-labelled Annexin-V followed by flow cytometric analysis. PI−Annexin-V− cells were regarded as viable cells. Data represent means±S.E.M. of data from 4–5 mice of each genotype
Loss of Bok does not accelerate lymphoma development in Eμ-myc transgenic mice
Somatically acquired copy number alterations involving the Bok gene have been detected in a range of human cancers at relatively high frequency,17 indicating that BOK may function as a tumour suppressor. Loss of pro-apoptotic BAX,21 BIM22 or PUMA,23, 24, 25 accelerated pre-B/B lymphoma development in Eμ-myc transgenic mice, which over-express the onco-protein c-MYC that deregulates cell proliferation in the B-lymphoid lineage under control of the IgH gene enhancer (Eμ).26
Interestingly, qPCR and western blot analyses revealed that pre-B cells from 5-week-old Eμ-myc mice (pre-leukaemic stage) had significantly lower levels of Bok mRNA and BOK protein, respectively, compared with pre-B cells from wt control animals (wt=0.0421, Eμ-myc=0.0201, P=0.0092; Supplementary Figure 4). Loss of one or both alleles of Bok did, however, not significantly affect pre-B/B lymphoma development in Eμ-myc transgenic mice (median lymphoma-free survival: Eμ-myc: 107 days; n=16, Eμ-myc/Bok+/−: 132 days; n=57, Eμ-myc/Bok−/−: 121 days; n=25) (Figure 8a). Moreover, post mortem analysis on sick, tumour-burdened animals revealed that spleen, thymus and lymph node weights, as well as total white blood cells counts were not significantly different between control Eμ-myc, Eμ-myc/Bok+/− and Eμ-myc/Bok−/− mice (Figures 8b–e), demonstrating that the loss of BOK had no impact on disease severity. Taken together, these results demonstrate that BOK does not function as a suppressor of c-MYC driven pre-B/B-cell lymphoma development.
Loss of Bok does not accelerate lymphomagenesis in Eμ-myc transgenic mice. (a) Kaplan–Meier survival curves showing lymphoma-free survival of control Eμ-myc, Eμ-myc/Bok+/− and Eμ-myc/Bok−/− mice. No statistically significant differences in survival were observed between mice of the different genotypes (P=0.303). The severity of disease (lymphoma) manifestation in mice of the three different genotypes was determined at time of death by measuring the weights of their (b) spleen, (c) thymus and (d) LN (combined mesenteric, axillary, brachial and inguinal) and determining (e) the numbers of leucocytes in their blood (WBC)
Discussion
BOK was originally discovered as a protein that bound to MCL-1 in a yeast 2-hybrid library screen of a rat ovarian gene expression library.12 On the basis that BOK shares most extensive amino-acid sequence similarity with BAX and BAK, it is widely believed that it functions in a BAX/BAK like manner, that is, in the activation of the effector phase of apoptosis that is characterised by MOMP and unleashing of the caspase cascade. However, BOK is one of the most poorly studied members of the wider BCL-2 family and there is no definitive understanding of its role in normal physiology or conditions of pathological stress. To determine the function of BOK, we performed an extensive analysis of its expression in diverse tissues and cell types, and examined the consequences of its loss in BOK-deficient mice.
Consistent with previous findings, we observed prominent Bok mRNA and protein levels in the ovaries and uterus. BOK was, however, also readily detected in many additional organs, such as the brain, spleen, lung and stomach. This indicates that BOK may have a wider role rather than being limited to the female reproductive system as previously anticipated.12 Further qPCR and western blot analyses showed that cells from Bax−/−, Bak−/− and even those from Bax−/−Bak−/− animals expressed similar levels of Bok mRNA and BOK protein as wt mice. These results indicate that loss of BAX or BAK, or even both pro-apoptotic multi-BH domain proteins do not exert pressure for a compensatory increase in Bok expression. This does, however, not exclude the possibility for functional overlap between BOK with BAX/BAK, given that despite the well-established functional overlap between BAX and BAK, Bax−/− cells express normal levels of BAK and Bak−/− cells express normal levels of BAX (e.g. Lindsten et al.7).
Earlier studies trying to define the function of Bok have employed over-expression systems in normal or transformed cells.12, 14 These experiments showed that BOK over-expression could trigger apoptosis either on its own or enhance apoptosis elicited by treatment with certain cytotoxic drugs. Our analysis of Bok-deficient mice represent to our knowledge the first investigation of the physiological roles of BOK during development and tissue homeostasis in adulthood. We did not recognise any major developmental abnormalities or phenotypic defects in newborn, adult or aged Bok−/− mice. Interestingly, Bok-deficient females, but not males, had significantly increased spleen and thymic weights compared with their wt counterparts. Although automated cell counting and FACS analysis did not reveal abnormally elevated numbers of total leukocytes or specific lymphoid/myeloid sub-populations in these tissues, we noticed a trend showing a mild increase across all cell populations that might partly account for this difference in weight. It is currently unclear why a sex-specific lymphoid organ weight increase is observed in our Bok−/− mice, but this might be owing to an indirect effect of hormonal regulation and/or hematopoietic cell extrinsic factors, such as the supporting tissue framework, extracellular matrix or water retention. Notably, it appears that BOK levels in the spleen and thymus are higher in stromal cells than hematopoietic ones.
Despite previous reports suggesting a pro-apoptotic function for BOK, we have yet to observe any developmental abnormalities in BOK-deficient mice indicative of defects in apoptosis. These findings are not unlike those from studies of mice lacking either BAK or BAX alone, which are generally normal and healthy with no major dysfunctions apart from sterility in Bax−/− males.7, 27 Moreover, the lymphoid and myeloid cells from Bax−/− or Bak−/− mice, like those from our Bok−/− animals (Figure 7), did not exhibit abnormally increased resistance to apoptotic stimuli in vitro, whereas Bax−/−Bak−/− lymphoid cells8 and fibroblasts7 were profoundly resistant to diverse cytotoxic stressors. These observations suggest that functional overlap may exist between BOK and BAX or/and BAK, and this can now be tested by generating Bok−/−Bax−/−, Bok−/−Bak−/− and eventually also Bok−/−Bax−/−Bak−/− animals. It is, however, also possible that BOK may have a function in a non-apoptotic process (not overlapping with the functions of BAX/BAK). If this was the case, the developmentally programmed cell deaths that still occur in Bax−/−Bak−/− mice may be safeguarded by a non-apoptotic cell death program, such as necroptosis.28, 29
It is also possible that the function of Bok may only manifest under specific circumstances and/or in limited cell types (i.e., those expressing the highest levels of BOK have not yet been examined by in vitro cell survival assays). For instance, on the basis of gene expression analysis, it has been postulated that Bok may be responsible for the death of placental trophoblast cells under hypoxic conditions in women suffering from pre-eclampsia.15, 16 It is noteworthy in this context that the promoter region of mouse and human Bok was found to contain two conserved binding sites for the hypoxia inducible transcription factor HIF-1α.30 Accordingly, it will be interesting to examine whether Bok is critical for the death of cells in response to pathological states that cause hypoxia, such as mouse models of stroke or cardiac dysfunction.
A global genomic screen of multiple human cancers found that the Bok gene is localised in a region that is frequently inactivated or deleted during tumourigenesis.17 Pertinently, earlier studies have shown that loss of the close relative Bax could accelerate lymphoma development in Eμ-myc transgenic mice21 as well as SV40 large T-antigen-induced development of brain31 or breast cancer.32 As Bok mRNA and BOK protein could be detected in non-transformed B lymphoid cells (from which Eμ-myc lymphomas arise),26 we examined whether its loss could accelerate Eμ-myc-induced lymphomagenesis. We found, however, that Bok deficiency had no impact on the rate of onset, overall incidence or severity of lymphomatous disease in this particular model. This may imply that Bok does not function as a tumour suppressor, and that therefore loss of a gene located near the Bok gene may account for the selection for somatically acquired copy number loss of this genomic region in human cancers.17 Alternatively, Bok may possibly function as a tumour suppressor within the context of certain oncogenic lesions (not those leading to c-MYC over-expression), for example, in cells where it is normally highly expressed, such as the brain or reproductive tissues. A role for BOK in suppressing the development of solid tumours could be addressed by crossing our Bok−/− mice with tumour prone transgenic, knock-out or spontaneous mutant mice (e.g., Apcmin mice), or by subjecting Bok−/− mice to protocols for chemical mutagen- or viral infection-induced tumourigenesis.
In summary, we conclude that Bok is expressed not only in female reproductive organs as reported previously,12 but in a considerably broader range of tissues and cell types. Moreover, our analyses of Bok-deficient mice so far have failed to define a critical function for this BAX/BAK-related Bcl-2 family member, perhaps indicating that its action is largely overlapping with that of those two proteins or that it may be restricted to specific conditions of stress in particular cell types. Our Bok−/− mice are made freely available for testing its role in such processes and pathological states.
Materials and Methods
Generation of Bok−/− mice
The Bok−/− mice were generated by gene targeting in embryonic stem (ES) cells. The targeting vector (Figure 2a) was prepared from C57BL/6 BAC DNA (RP23-262D13) (Australian Genome Research Facility, Melbourne, Australia) by flanking the 1.25 kb targeted region encompassing half of exon 1 and exon 2 of the Bok gene with LoxP sites, and introducing a Pgk−1 promoter-driven hygromycin resistance cassette flanked by FRT sites. The 5′- and 3′-arms were 2.54 kb and 2.37 kb in length, respectively. A total of 20 μg of linearised targeting vector was electroporated into C57BL/6-derived Bruce4 ES cells. Hygromycin-resistant ES clones were screened by Southern blotting with 5′ and 3′ external probes to discriminate between the wt and floxed alleles. DNA from correctly targeted clones was further tested using hybridisation to a PGKhygro-specific probe to confirm single construct integration.
Two recombinant ES clones carrying the targeted mutation in the Bok gene were injected into C57BL/6 blastocysts, which were subsequently implanted into pseudo-pregnant BALB/c mice. Chimaeric offspring with significant contribution of black fur were crossed to C57BL/6 mice to generate floxed Bok lines, denoted Bokfl 535 and Bokfl 536. To check for the targeted allele, genotyping was performed with forward (5′-CGGGTTTGAATGGAAGGGTC-3′) and reverse primers (5′-TGTTCCCATGGTGCTACATCC-3′) to obtain a 150 and 200 bp product for the wt and floxed allele, respectively.
To generate Bok-deficient mice, heterozygous floxed males (Bokfl/+) were crossed to C57BL/6 deleter females (Cre+/tg)33 to achieve Cre recombinase-mediated excision of the targeted region in all cells. Offspring with a deleted Bok allele (Bok+/−) were backcrossed for three generations with C57BL/6 mice and subsequent intercrosses of Bok+/− mice were set up to generate Bok+/+, Bok+/− and Bok−/− mice for analysis. Genotyping of Bok-deficient mice (lines del535 and del536) was performed using a three-primer PCR reaction, utilising a common forward primer (5′-CGGGTTTGAATGGAAGGGTC-3′) in combination with two reverse primers (5′-TGTTCCCATGGTGCTACATCC-3′ and 5′-GAGCTAGCTAGCTATGTGTG-3′). Products of 150 and 383 bp were observed when the reaction mixture was separated on a 2% agarose gel, corresponding to the wt and Bok mutant allele, respectively. PCR primers for the detection of Cre were 5′-CTGACCGTACACCAAAATTTGCCT-3′ (forward) and 5′-GATAATCGCGAACATCTTCAGGTTC-3′ (reverse), amplifying a 210 bp product. All experiments with mice were performed according to the guidelines of the animal ethics committees of the Melbourne Health Research Directorate.
Quantitative PCR analysis
RNA was isolated from sorted cells and tissues by homogenisation after addition of TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA was quantified using the NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) technology and 1 μg was used for cDNA synthesis using the SuperScript III First-Strand Synthesis SuperMix Kit (Invitrogen) in a 20-μl reaction volume. PCR amplifications were performed with a 7900HT thermal cycler (Applied Biosystems, Carlsbad, CA, USA) using 1 μl of cDNA and 1 μl of Taqman primers (Applied Biosystems) specific for bok (Mm00479421_m1) or ATP5a1 (Mm01257366_m1) in a 96-well plate, under standard running conditions as suggested by the manufacturer. Three replicates of each reaction were performed. All qPCR data were subsequently analysed using the 2−ΔΔCT method,34 where Atp5α1 was assigned as the housekeeping gene, and a specific brain sample was designated as the reference sample in all qPCR reactions. This allowed us to compare data between different experiments, where Bok mRNA levels in all samples are presented as the fold difference relative to the same reference sample.
Western blot analysis
Total protein extracts from mouse tissues were prepared in modified RIPA buffer (50 mM Tris/HCl pH 8.0, 100 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 5 mM EDTA) complemented with protease inhibitors (Roche (Basel, Switzerland) COMPLETE protease inhibitor cocktail plus 1 μg/ml pepstatin). Protein concentration was determined using a BCA assay (Thermo Fisher Scientific) and 30 μg of total protein per extract were resolved by SDS-PAGE. Western blotting was performed using rabbit polyclonal antibodies raised against amino acids 19–32 of mBOK and, as a loading control, a mouse monoclonal antibody to GAPDH (clone 6C5, Millipore, Billerica, MA, USA).
Histology
Mouse organs were fixed in either 10% buffered formalin or Bouin's solution and subsequently embedded in paraffin. Histological sections were prepared and stained with haematoxylin/eosin. Whole brains were collected into Bouin's fixative, embedded in paraffin and serial sections prepared for staining with crystal violet dye. Organ sections (including those of brains) were examined with a compound microscope (Zeiss, Oberkochen, Germany) and light microscope (Zeiss), respectively, and photographed with a digital camera (AxioCam HRc Zeiss). From serially sectioned ovaries (n=3–6 wt or Bok−/−), follicles containing an oocyte were counted in six equally spaced sections across each ovary and expressed as the mean±S.E.M. per 104 μm2 (mm2 for PN11).
Immunofluorescence staining and flow cytometric analysis
Antibodies used for immunofluorescence staining and FACS analysis or cell sorting included anti-CD4 (YTA321 or H129), anti-CD8 (YTS169), anti-B220 (RA3-6B2), anti-IgM (5.1), anti-IgD (1126C), anti-c-Kit (ACK-4), anti-Mac-1 (MI/70), anti-Gr-1 (RB6-8C5) and anti-Ter119, conjugated to fluorescein isothiocyanate (FITC; Caltag, Buckingham, UK), phycoerythrin (R-PE) or allophycocyanin (APC; both from Prozyme, Hayward, CA, USA). FACS analysis of hematopoietic tissues (spleen, bone marrow, thymus, lymph nodes and blood) was carried out on an LSR1 (Becton Dickinson, Franklin Lakes, NJ, USA) machine and cell sorting was performed using a MoFlo (Cytomation, Boulder, CO, USA) high-speed cell sorter.
Hematopoietic analysis
Peripheral blood was isolated from 8–12-week-old mice by cardiac puncture after euthanasia, and haematological analysis was performed using the ADVIA automated haematology system (Bayer, Tarrytown, NY, USA). Single-cell suspensions were prepared from the thymus, spleen, LN and BM (two tibias and two femurs), and viable cell numbers were enumerated with the automated CASY counter. The cell subset composition of these lymphoid organs was determined by staining with fluorochrome (R-PE, APC or FITC)-conjugated monoclonal antibodies specific to cell lineage markers (see above) and FACS analysis.
Cell culture and cell survival assays
Lymphoid and myeloid cell subsets were isolated by FACS sorting based on their surface marker expression profiles as described above. Immature CD4+CD8+ (double-positive, DP) thymocytes were sorted from the thymus, mature CD4+CD8−, CD4−CD8+ T cells and mature B220+ B cells were sorted from the lymph nodes, and Gr-1+Mac-1+ granulocytes were sorted from the bone marrow. All cells were cultured at 37 °C in a humidified 10% CO2 incubator in high-glucose DMEM supplemented with 10% heat-inactivated foetal calf serum (JRH Biosciences, Lenexa, KS, USA), 50 μM 2-mercaptoethanol, and 100 μM asparagine. Sorted cells were cultured in 96-well flat bottom plates at a density ranging between 3–5 × 105 cells/ml. Apoptotic stimuli applied included dexamethasone (1 μM), etoposide (1 μM), ABT-737 (240 nM) or FLAG-tagged FasL (100 ng/ml; Enzo Life Sciences, Farmingdale, NY, USA) cross-linked with anti-Flag M2 antibody (2 μg/ml; Sigma, St. Louis, MO, USA). Death by cytokine deprivation was mimicked by plating cells in DMEM +10% FCS without the addition of cytokines. To assay for cell survival, cells were stained with 5 μg/ml of propidium iodide (PI) plus FITC-conjugated annexin-V and analysed by flow cytometry at various time intervals. Viable cells were defined as those that were not stained by either PI or FITC-annexin-V (FITC−PI−).
Statistical analysis
All statistical analyses were performed using the two-tailed Student's t-test, and P-values <0.5, were considered significant.
Abbreviations
- APC:
-
allophycocyanin
- ATP5a1:
-
ATP synthase H+ transporting mitochondrial F1 complex alpha subunit 1
- bp:
-
basepair
- BM:
-
bone marrow
- cDNA:
-
complementary deoxyribonucleic acid
- CC:
-
corpus callosum
- Ctx:
-
cerebral cortex
- ddCT:
-
delta-delta CT
- DMEM:
-
Dulbecco's modified Eagle medium
- DNA:
-
deoxyribonucleic acid
- EDTA:
-
ethylenediaminetetraacetic acid
- ESC:
-
embryonic stem cells
- FACS:
-
fluorescence activated cell sorting
- FCS:
-
fetal calf serum
- FITC:
-
fluorescein isothiocyanate
- FasL:
-
Fas ligand
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- h:
-
hour
- HCl:
-
hydrochloric acid
- HIF1α:
-
hypoxia-inducible factor 1 α subunit
- LN:
-
lymph nodes
- mac:
-
macrophages
- neu:
-
neutrophils
- mg:
-
milligram
- μg:
-
microgram
- mL:
-
milliliter
- μL:
-
microlitre
- mM:
-
millimolar
- kDa:
-
kilo Dalton
- MOMP:
-
mitochondrial outer membrane permeabilisation
- mRNA:
-
messenger ribonucleic acid
- NaCl:
-
sodium chloride
- PI:
-
propidium iodide
- qPCR:
-
quantitative polymerase chain reaction
- R-PE:
-
red phycoerythrin
- SCNA:
-
somatically acquired copy number alterations
- SD:
-
standard deviation
- SDS-PAGE:
-
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- SEM:
-
standard error of the mean
- Se:
-
septum
- sIg:
-
surface immunoglobulin
- Str:
-
Striatum
- SV40:
-
simian virus 40
- Ter119+ cells:
-
erythroid cells expressing the Ter119 lineage marker
- WBC:
-
white blood cells
- wt:
-
wild-type
References
Youle RJ, Strasser A . The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 2008; 9: 47–59.
Chipuk JE, Green DR . How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 2008; 18: 157–164.
Huang DCS, Strasser A . BH3-only proteins – essential initiators of apoptotic cell death. Cell 2000; 103: 839–842.
Letai AG . Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer 2008; 8: 121–132.
Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 2007; 315: 856–859.
Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O’Reilly LA et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol 2009; 186: 355–362.
Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389–1399.
Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB . Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol 2002; 3: 932–939.
Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB . BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 2001; 15: 1481–1486.
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T et al. BCL-2, BCL-xL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 2001; 8: 705–711.
Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S . Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 1995; 14: 6136–6147.
Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJW . Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci USA 1997; 94: 12401–12406.
Hsu SY, Hsueh AJW . A splicing variant of the Bcl-2 member Bok with a truncated BH3 domain induces apoptosis but does not dimerize with antiapoptotic Bcl-2 proteins in vitro. J Biol Chem 1998; 273: 30139–30146.
Inohara N, Ekhterae D, Garcia I, Carrio R, Merino J, Merry A et al. Mtd, a novel Bcl-2 family member activates apoptosis in the absence of heterodimerization with Bcl-2 and Bcl-xL . J Biol Chem 1998; 273: 8705–8710.
Ray JE, Garcia J, Jurisicova A, Caniggia I . Mtd/Bok takes a swing: proapoptotic Mtd/Bok regulates trophoblast cell proliferation during human placental development and in preeclampsia. Cell Death Differ 2010; 17: 846–859.
Soleymanlou N, Wu Y, Wang JX, Todros T, Ietta F, Jurisicova A et al. A novel Mtd splice isoform is responsible for trophoblast cell death in pre-eclampsia. Cell Death Differ 2005; 12: 441–452.
Beroukhim R, Mermel C, Porter D, Wei G, Raychaudhuri S, Donovan J et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463: 899–905.
Deckwerth TL, Elliott JL, Knudson CM, Johnson Jr EM, Snider WD, Korsmeyer SJ . BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 1996; 17: 401–411.
Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 1999; 286: 1735–1738.
Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 2003; 302: 1036–1038.
Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL . Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol 2001; 21: 7653–7662.
Egle A, Harris AW, Bouillet P, Cory S . Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA 2004; 101: 6164–6169.
Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW . Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA 2004; 101: 9333–9338.
Garrison SP, Jeffers JR, Yang C, Nilsson JA, Hall MA, Rehg JE et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol 2008; 28: 5391–5402.
Michalak EM, Jansen ES, Happo L, Cragg MS, Tai L, Smyth GK et al. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ 2009; 16: 684–696.
Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 1985; 318: 533–538.
Knudson CM, Tung KSK, Tourtellotte WG, Brown GAJ, Korsmeyer SJ . Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995; 270: 96–99.
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE . Cell death. N Engl J Med 2009; 361: 1570–1583.
Degterev A, Yuan J . Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol 2008; 9: 378–390.
Gao S, Fu W, Durrenberger M, De Geyter C, Zhang H . Membrane translocation and oligomerization of hBok are triggered in response to apoptotic stimuli and Bnip3. Cell Mol Life Sci 2005; 62: 1015–1024.
Yin CY, Knudson CM, Korsmeyer SJ, Van Dyke T . Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 1997; 385: 637–640.
Shibata MA, Liu ML, Knudson MC, Shibata E, Yoshidome K, Bandey T et al. Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40-TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. EMBO J 1999; 18: 2692–2701.
Schwenk F, Baron U, Rajewsky K . A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 1995; 23: 5080–5081.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Acknowledgements
We thank Drs JM Adams, S Cory, DCS Huang, D Gray, M Herold and G Kelly for mice, reagents and advice; K Vella, G Siciliano, D Cooper, N Iannarella and J Coughlin for animal care; J Corbin for automated blood analysis; B Helbert and C Young for genotyping; E Tsui for histological preparations; Dr. F Battye and his team for cell sorting; D Quilici, T Nikolaou and G Thomas for irradiation and D Bachmann for expert technical assistance. This work was supported by grants and fellowships from the Leukemia Research Foundation (to FK), the National Health and Medical Research Council (Program Grant no. 461221, NHMRC Australia Fellowship), the NIH (CA43540), the Leukemia and Lymphoma Society (SCOR grant no. 7413), the Swiss National Science Foundation (to TK, PP0033_119203) and operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by C Borner
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Ke, F., Voss, A., Kerr, J. et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ 19, 915–925 (2012). https://doi.org/10.1038/cdd.2011.210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2011.210
Keywords
This article is cited by
-
Targeting the apoptosis pathway to treat tumours of the paediatric nervous system
Cell Death & Disease (2022)
-
Mitochondria as multifaceted regulators of cell death
Nature Reviews Molecular Cell Biology (2020)
-
Glucocorticoids can induce BIM to trigger apoptosis in the absence of BAX and BAK1
Cell Death & Disease (2020)
-
Modeling the function of BAX and BAK in early human brain development using iPSC-derived systems
Cell Death & Disease (2020)
-
Bok regulates mitochondrial fusion and morphology
Cell Death & Differentiation (2019)