Abstract
p53 is a key regulator of cell growth and death by controlling cell cycle progression and apoptosis under conditions of stress such as DNA damage or oncogenic stimulation. As these processes are critical for cell function and inhibition of tumor development, p53 regulatory pathways are strictly monitored in cells. Recently, it was recognized that nucleolar proteins, including nucleophosmin/B23, ribosomal protein L11, and alternate reading frame (ARF), form the nucleolus-ARF-murine double minute 2 (MDM2) axis in p53 regulatory pathways, which increases p53 stability by suppressing the activity of MDM2. In this work, we show that nucleolar protein glioma tumor-suppressor candidate region gene 2 (GLTSCR2) translocates to the nucleoplasm under ribosomal stress, where it interacts with and stabilizes p53 and inhibits cell cycle progression without the involvement of the major upstream p53 regulator, ARF. Furthermore, ectopic expression of GLTSCR2 significantly suppressed growth of cancer cells in a xenograft animal model via p53-dependent pathway. Our data identify GLTSCR2 as a new member of the nucleolus–nucleoplasmic axis for p53 regulation. ARF-independent direct regulation of p53 by GLTSCR2 may be a key mechanism and therapeutic target for cell death or growth inhibition when nucleolus-ARF-p53 pathways are inactivated by genetic or epigenetic modifications of ARF, which are the second most common types of genetic change observed in human cancers.
Similar content being viewed by others
Main
The p53 tumor suppressor protein is a key regulator of cell growth and death. p53 induces cell cycle arrest, apoptosis, and DNA repair by downstream gene activation in response to a variety of stresses.1, 2 Therefore, p53 is a central target of inactivation in the process of neoplastic transformation, and mutations of its core domain are frequently encountered in human cancers.3 In unstressed physiological states, cellular p53 is maintained at low levels by its primary negative regulator, murine double minute 2 (MDM2).4, 5 Under conditions of stress, however, p53 is stabilized and activated by the suppression of MDM2 activity through diverse transcriptional and/or translational pathways.6 As this process is critical not only for the maintenance of normal cellular functions but also for the prevention of pathological conditions caused by dysregulated apoptosis or cell cycle progression, all steps involved in p53 regulation are strictly monitored by the sensing and executing machineries in cells.
Recently, new nucleolus-nucleoplasm networks of proteins that regulate p53 stability have been recognized.7 These protein axes include nucleophosmin (NPM)/B23,8 ribosomal protein (RP) L11 (RPL11),9 RPL23,10 RPL5,11 and nucleostemin (NS),12 and mostly interact with MDM2 to inhibit MDM2-mediated p53 degradation through alternative reading frame (ARF)-dependent pathways. ARF is a nucleolar–nucleoplasmic shuttling protein that protects cells from oncogenic transformation.13 The key molecular pathway activated by ARF for tumor suppression propagates through p53-dependent cell cycle arrest or apoptosis. ARF inactivates and degrades MDM2 by direct physical interactions and thus inhibits MDM2-dependent degradation of p53, which in turn activates p53-dependent tumor suppressive pathways.14, 15 The importance of ARF in tumor suppression is supported by the fact that mutational inactivation of ARF is the second most frequent genetic alteration encountered in human cancers.16 On the other hand, the high frequency of ARF inactivation in tumors may paradoxically introduce the possibility that cells possess alternative mechanisms for the maintenance of p53 stability when the ARF-MDM2-p53 regulatory pathway is inactivated by genetic or epigenetic events.
Glioma tumor-suppressor candidate region gene 2 (GLTSCR2) is one of the nucleolar proteins. Previously, we and others have shown that GLTSCR2 may be a tumor suppressor owing to the following observations: (i) it is frequently deleted or downregulated in human cancers, especially in brain tumors;17, 18 (ii) it physically interacts with and phosphorylates the tumor suppressor, phosphatase and tensin homolog;19 and (iii) overexpression of GLTSCR2 induces apoptotic cell death.20 However, the biological function of GLTSCR2 in the process of tumor cell death and underlying molecular mechanisms are largely unknown. Although investigating the role of GLTSCR2 in inhibition of tumor cell growth, we found that nucleoplasm-mobilized GLTSCR2 stabilizes p53 through direct protein–protein interaction, thereby increasing cellular levels of p53 and suppressing in vitro and in vivo tumor growth. Unlike other members of the nucleolus-MDM2-p53 axis, GLTSCR2 directly stabilizes p53 in ARF-independent pathways. Our data indicate that GLTSCR2 is a key upstream regulator of p53 and could be an effective therapeutic target for cell death induction in cancers with inactivated nucleolus-ARF-p53 axes.
Results
Controlled overexpression of GLTSCR2 reduces cell proliferation
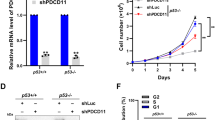
Previously, we and others reported that GLTSCR2 is involved in the death and transformation of tumor cells.17, 18, 19, 20 To further investigate the role of GLTSCR2 in tumor cell growth and the underlying molecular mechanisms we constructed an adenoviral system that expresses green fluorescence protein (GFP)-tagged GLTSCR2 (Ad-GLT/GFP), in which the expression level is regulated by the concentration of doxycycline (Tet-Off system), to avoid non-physiological high expression levels observed with transient transfection of plasmids. A549 lung adenocarcinoma cells were infected with Ad-GLT/GFP or control virus (Ad-GFP) in the absence or presence of different concentrations of doxycyclin, and growth rates were determined by counting the number of cells. Induction of GLTSCR2 expression suppressed tumor cell growth in a concentration-dependent manner (Figure 1a) and inhibited cell cycle progression (Figure 1b). The expressions of ectopic GLTSCR2 according to time points or doxycycline concentrations are shown in Supplementary Figures S1a and b. We performed western blot analyses for proteins associated with cell cycle progression, including p53, p21, p16INK4, and p27 to explore the molecular mechanisms of cell growth inhibition by GLTSCR2 overexpression. Among them, we observed an increase in expression of p53 and its downstream effector molecule, p21 (Figure 1c and Supplementary Figures S1a and b), suggesting the involvement of p53 in GLTSCR2-induced suppression of tumor cell growth. GLTSCR2-dependent p53 expression was confirmed in cell types including HCT116 (p53+/+) and keratinocytes (Supplementary Figure S1c). To further determine whether p53 protein level is regulated by GLTSCR2, we downregulated GLTSCR2 expression by infecting A549 cells with lentiviral small hairpin RNA targeting GLTSCR2 (Lenti-shGLT). Lenti-shGLT reduced GLTSCR2 protein levels with 80–90% knockdown efficiency compared with the sample treated with scrambled shRNA (Lenti-shScr; Figure 1d, top panel). p53 and p21 protein levels were reduced in A549 cells with GLTSCR2 knockdown compared with control cells (Figure 1d, lower three panels). Downregulation of p53 and p21 by GLTSCR2 knockdown was also observed in HCT116 (p53+/+) cells (Supplementary Figure S1d). In addition, a significant decrease of BrdU incorporation in HCT116 (p53+/+), but not in HCT116 (p53−/−), by GLTSCR2 overexpression indicates that GLTSCR2 suppress cell growth in p53-dependent manner. Taken together, our results suggest that GLTSCR2 reduces tumor cell growth with involvement of the p53 pathway.
GLTSCR2 inhibits cell proliferation and cell cycle progression with p53 upregulation. (a) A549 cells were infected with Ad-GLT/GFP or Ad-GFP (control) with the indicated concentrations of doxycyclin. Cell proliferation rates were assessed by counting cell numbers up to 96 h. The initial cell number was 1 × 105. Data are shown as the fold increase of three independent experiments (mean±S.D.). (b) The cell cycle profile analysis of cells infected by Ad-GLT/GFP for 4 days with the indicated concentration of doxycyclin. (c) A549 cells were infected with Ad-GLT/GFP or Ad-GFP. After 24-h infection, cells were untreated or treated with decreasing amounts of doxycyclin (50 ng/ml, 20 ng/ml, 10 ng/ml, and 0 ng/ml, respectively) for an additional 24 h. Cells were harvested and lysates were subjected to western blotting using the indicated antibodies (upper three panels) after normalization to GAPDH (lowermost panel); ecto, ectopic expression; endo, endogenous expression. (d) Knockdown of GLTSCR2 decreases the expression of p53 and downstream p21 protein. A549 cells were infected by GFP-tagged lentivirus targeting GLTSCR2 (Lenti-shGLT) or with scrambled (Lenti-shScr) shRNA. Lysates from Lenti-shGLT or Lenti-shScr cells were subjected to western blotting for evaluation of GLTSCR2 knockdown, or p53 and p21 expression, after normalization to GAPDH. (e) HCT116 (p53+/+) and HCT116 (p53−/−) cells were infected by Ad-GFP or Ad-GLT/GFP for 48 h and then pulsed with BrdU for 1 h before fixation. Cells with BrdU immunofluorescence were counted. Data represent percentage of cells with BrdU uptake (mean±S.D.) of three independent experiments. *P<0.05; NS, not significant
GLTSCR2 interacts with p53
To elucidate the molecular mechanisms of p53 upregulation, we initially investigated whether GLTSCR2 physically interacts with p53 or MDM2 because other nucleolar proteins such as ARF,15 NPM,21 and NS22 are associated with p53 or its primary negative regulator MDM2 to increase the cellular level of p53. p53-deficient H1299 cells were co-transfected with GFP-tagged GLTSCR2 (GFP-GLT) and V5-tagged p53 (V5-p53) expression plasmids, followed by a co-immunoprecipitation and immunoblotting assay. Using an anti-GFP antibody for immunoprecipitation, GFP-GLTSCR2 was found to be associated with V5-p53 (Figure 2a, upper panel), whereas GFP alone did not (Figure 2b). Conversely, V5-p53 was specifically immunoprecipitated with GFP-GLTSCR2 by the anti-V5 antibody (Figure 2a, middle panel). Next, to determine whether endogenous GLTSCR2 and p53 also interact with each other, cell lysates from A549 cells with wild-type p53 were used in a similar assay. As shown in Figure 2c, endogenous p53 and GLTSCR2 were co-immunoprecipitated by antibodies against either of these two proteins. These results demonstrate that GLTSCR2 interacts with p53 in cells. To determine whether GLTSCR2-p53 binding is direct, a glutathione S-trasnferase (GST) pull down assay was performed using GST-tagged GLTSCR2 and recombinant p53 purified from bacteria. GST-GLTSCR2, but not GST alone, interacted with p53 (Figure 2d). We further investigated whether GLTSCR2 interacts with MDM2; however, MDM2 was not associated with GLTSCR2 in immunoprecipitation assay (Supplementary Figure S2). Taken together, our results demonstrate that GLTSCR2 interacts with p53 in cells and in vitro.
Interaction of GLTSCR2 with p53 in cells and in vitro. (a) Ectopically expressed GLTSCR2 and p53 interact with each other in vivo. H1299 cells were singly transfected or co-transfected with GFP-tagged GLTSCR2 (GFP-GLT) and V5-tagged p53 (V5-p53) expression plasmids as indicated. Cell lysates were immunoprecipitated using anti-GFP (upper panel) or anti-V5 (middle panel) antibody, and western blotting was performed using anti-GFP or anti-V5 antibody. A 10% loading control is shown in the lower panel. (b) Cells were transfected with empty GFP plasmid instead of the GFP-GLTSCR2 expression plasmid and an immunoprecipitation assay was performed as in (a) to demonstrate the specificity of interaction between GLTSCR2 and p53. (c) Endogenous GLTSCR2 and p53 interact with each other in vivo. Cell lysates from A549 cells were immunoprecipitated with anti-GLTSCR2 (upper panel) or anti-p53 (lower panel) antibody, and western blotting was conducted with the indicated antibodies. (d) GLTSCR2 binds to p53 in vitro. A total of 200 ng of purified GST alone or GST-tagged GLTSCR2 immobilized on glutathione beads was incubated with 200 ng of the recombinant p53 protein purified from bacteria. Bound p53 was detected with anti-p53 antibody (upper panel). Recombinant GST, GST-GLTSCR2, and p53 proteins were visualized by Coomassie blue staining (lower panel)
p53–GLTSCR2 interaction involves the tetramerization domain (TTD) of p53 and N63–149 or C347–432 of GLTSCR2
To map the GLTSCR2-binding domain of p53, we generated a set of deletion mutants of p53 that correspond to the transactivation (TAD), DNA binding (DBD), TTD, and nuclear localization signal (NLS) domains (Figure 3a). Co-immunoprecipitation assays of GFP-tagged wild-type or mutant p53 and V5-tagged GLTSCR2 showed that TAD, DBD, and NLS lost binding capability to GLTSCR2 (Figure 3b). This suggests that the TTD of p53 is important for GLTSCR2–p53 interaction. Next, to define the p53-binding domain of GLTSCR2, deletion truncates were made on GLTSCR2 (Figure 3c), and similar assays were performed using anti-GFP antibody. As shown in Figure 3d, deletion of both N63–149 residues and C347–432 residues abolished the binding of p53 to GLTSCR2. To further show that N63–149 and C347–432 residues are pivotal in GLTSCR2–p53 interaction, we generated additional truncation mutants of GLTSCR2, M6, and M7 (Supplementary Figure S3a) and performed co-immunoprecipitation assays with V5-tagged p53. These mutants were specifically immunoprecipitated with p53 (Supplementary Figures S3b and c). Together, these findings indicate that the TTD of p53 and either the N63–149 or C347–432 residues of GLTSCR2 are important for the physical interactions of p53 and GLTSCR2.
Mapping of the domains responsible for the interaction between p53 and GLTSCR2. (a) Schematic diagram of p53 and its deletion mutants. (b) The TTD of p53 is important for interaction with GLTSCR2. A549 cells were transfected with GFP-tagged wild-type or mutant p53, together with V5-tagged GLTSCR2 plasmid. Cell lysates were immunoprecipitated with anti-V5 antibody, followed by western blotting using the indicated antibodies (upper and middle panels). Expression level of input GFP–p53 fusion proteins was shown in lower panel. (c) Schematic diagram of GLTSCR2 and its deletion mutants. (d) C-terminal 63–149 or N-terminal 347–432 residues of GLTSCR2 bind to p53. A549 cells were transfected with GFP-tagged wild-type or mutant GLTSCR2, together with V5-tagged wild-type p53 plasmid. Cell lysates were immunoprecipitated with anti-GFP antibody, followed by western blotting using the indicated antibodies (upper and middle panels). Expression level of input GFP–GLTSCR2 fusion proteins was shown in lower panel
GLTSCR2 stabilizes p53 through ARF-independent direct interaction
To investigate whether p53 induction by GLTSCR2 was caused by GLTSCR2–p53 interactions, ARF-deficient A549 cells (Figure 4a) were transfected with a series of GLTSCR2 deletion mutants shown in Figure 3c and p53 levels were determined. We used ARF-deficient A549 cells because nucleolar proteins, including NPM and NS,8, 12 upregulate p53 through the ARF-MDM2 pathways. Interestingly, GLTSCR2 deletion mutants retained the ability to induce p53 protein with the exception of M2 and M5 (Figure 4b), which lost p53-binding ability (Figure 3d). These data, together with semiquantitative reverse transcription (RT)-PCR results showing nonsignificant alterations in p53 mRNA levels (Supplementary Figure S4), suggest that the interaction of GLTSCR2 with p53 is essential for translational upregulation of p53. Next, we measured the protein stability of p53 by a cycloheximide chase assay in p53-deficient H1299 cells. Ectopically expressed p53 was degraded more slowly in GLTSCR2-overexpressing cells than in GFP-expressing control cells or p53-non-binding M5-expressing cells (Figures 4c and d). These findings were confirmed by the GLTSCR2 knockdown approach. Protein degradation of p53 was increased in Lenti-shGLT-infected cells compared with Lenti-shScr-infected cells (Figures 4e and f). Next, we determined whether this stabilization of p53 is owing to the inhibition of ubiquitination of p53 because MDM2-mediated ubiquitin-proteasome pathway is a primary factor in p53 degradation.6 H1299 cells were transfected with indicated expression plasmids, and treated with the proteasome inhibitor, MG132 (20 μM), for 6 h. As shown in Figure 4g, overexpression of GLTSCR2, but not the p53-non-binding mutant, M5, inhibited the MDM2-mediated ubiquitination of p53. Taken together, our results demonstrate that physical interaction of GLTSCR2 with p53 increased the stability of p53 by the inhibition of ubiquitination.
GLTSCR2 enhances p53 stability through ARF-independent direct physical interaction with p53. (a) Western blot image for the detection of ARF expression from HeLa, H1299, and A549 cells. (b) Physical interaction between GLTSCR2 and p53 is crucial for p53 upregulation. A549 cells were transfected with wild-type or the indicated mutant GLTSCR2 plasmids (1 μg/106 cells). After 36 h, cells were harvested, lysed, and subjected to western blotting for the determination of p53 expression level after normalization to GAPDH; M, mock transfected. (c) A549 cells were transfected with mock-GFP (GFP), wild-type GLTSCR2-GFP (GLT-GFP), or mutant GLTSCR2-GFP (M2-GFP) plasmids (1 μg/106 cells) for 36 h. After treatment with 200 μg/ml cycloheximide, cell lysates were prepared at 20–30-min intervals from 0 to 2 h. The amount of p53 was determined using western blotting after normalization to GAPDH. Plot is densitometric quantitation of the cycloheximide chase assay. Data represent percentages of p53 intensity of three independent experiments (mean±S.D.) compared with that at the 0 time point. (d) Representative western blot images of cycloheximide chase assay. (e) A p53 degradation assay was performed using GLTSCR2-knocked down A549 cells (shGLT) or control cells (shScr) as in (c). The amount of p53 was determined using western blotting after normalization to GAPDH. Plot is densitometric quantitation of the cycloheximide chase assay. Data represent percentages of p53 intensity compared with that at the 0 time point. (f) Representative western blot images of cycloheximide chase assay in GLTSCR2-knocked down A549 cells (shGLT) or control cells (shScr). (g) GLTSCR2 decreases the ubiquitination of p53. H1299 cells were transfected with indicated plasmids. Cells were further treated with MG132 (20 μM) for 6 h, and cell lysates were passed through the columns packed with polyubiquitin affinity resin. Eluted proteins were subjected to western blot using anti-p53 antibody (upper panel). Ubiquitylated p53 (Ub-p53) is indicated. The expression of total p53, MDM2, and wild-type or mutant GLTSCR2 is shown in lower three panels
GLTSCR2 increases tetramerization of p53
Binding assays showed that the TTD of p53 is important for the interaction with GLTSCR2 (Figure 3b). The TTD of p53 is required for efficient site-specific DNA binding and contributes to the ability of p53 to activate transcription.23 In addition, disruption of p53 tetramerization exposes a nuclear export signal that stimulates Crm1-dependent exclusion of p53 from the nucleus.24 Therefore, we examined whether GLTSCR2 affects the tetramerization status of p53 in addition to its stabilization. Initially, we determined the phosphorylation status of p53 in GLTSCR2-overexpressing A549 cells. A549 cells were transfected with empty GFP or GFP-GLT, and the phosphorylation status of endogenous p53 was evaluated using the p53 sampler kit antibody after the indicated times. As shown in Figure 5a, overexpression of GFP-GLT, but not GFP alone, induced phosphorylation at Ser9, Ser37, and Ser392 of p53. As reported previously, phosphorylation at these sites is important for the tetramerization and transcriptional activity of p53.23, 25, 26 Next, we performed immunoblotting using glutaraldehyde cross-linked cell lysates to determine the oligomerization status. Cells were transfected with GFP, wild-type GLTSCR2 (WT), M3, M4, or M5 mutant plasmid, or infected with Lenti-shGLT, and lysed after treatment with 0.05% glutaraldehyde. Immunoblotting showed that overexpression of GLTSCR2 resulted in an increase of tetramer form of p53, compared with overexpression of M5 or GFP alone (Figure 5b). Overexpression of M3 or M4, which contains only one p53-binding motif, increased the tetramer form of p53, but less than wild-type GLTSCR2. However, downregulation of GLTSCR2 by shGLT did not change the ratio of tetramer form to total p53. Our data suggest that physical interaction of GLTSCR2 and p53 is important for tetramerization of p53. To further show that DNA-binding affinity of p53 was increased by GLTSCR2 we performed chromosome immunoprecipitation (ChIP). GLTSCR2 increased the DNA-binding affinity of p53 to p21 promoter while p53-non-binding mutant M5, which did not increase tetramerization of p53, failed to enhance binding affinity (Figure 5c). It was previously reported that tetrameric p53 is efficiently ubiquitinated by MDM2,27 suggesting higher binding affinity of the tetrameric form to MDM2. We performed co-immunoprecipitation experiments to examine whether GLTSCR2 affects binding affinity between p53 and MDM2. Contrary to previous reports, we did not observe significant changes in binding affinity between p53 and MDM2 (Supplementary Figure S5).
GLTSCR2 induces oligomerization of p53. (a) A549 cells were untreated or transfected with GFP-tagged GLTSCR2 expressing (GFP-GLT) or mock control vector (GFP) as indicated. After transfection for 12 and 24 h, cells were harvested and their lysates were subjected to western blotting for the detection of p53 phosphorylation at the indicated sites using indicated anti-phospho-p53 antibodies. Then, the blots were reprobed with anti-p53 antibody detecting total p53. (b) Cells were untransfected or transfected with empty GFP plasmid (GFP), wild-type (WT) or mutant GLTSCR2 (M3, M4, and M5)-expressing plasmid, or shRNA targeted to GLTSCR2 (shGLT) as indicated. After 24 h (GFP, WT, M3, M4, and M5) or 48 h (shGLT), cell lysates were obtained, treated with glutaraldehyde at a final concentration of 0.05% at room temperature for 20 min, and subjected to western blotting for the detection of monomer, dimer, and tetramer forms of p53. The histogram shows the percentage of the tetramer form in each sample determined by densitometric analysis of three independent experiments. Data are shown as the percentage of tetramer form of three independent experiments (mean±S.D.). *P<0.05. The representative western blots for p53 oligomer with glutaraldehyde treatment (upper panel) or p53 without glutaraldehyde treatment (middle panel) after normalization to GAPDH (lower panel) were presented. (c) Cells were transfected with GFP, GFP-GLT, or M5 mutant (GFP-M5) plasmid for 24 h, treated with formaldehyde, and then sonicated. The cross-linked DNA–protein complex was immunoprecipitated by anti-GFP (α-GFP) or anti-p53 (α-p53) antibody, followed by PCR amplification for p21 promoter. Immunoprecipitates by IgG served as negative controls. Input chromatin (Input) represents a part of the sonicated chromatin before immunoprecipitation. A PCR for the p21 non-specific site (NS) and GAPDH promoter were performed as a negative control
Nucleoplasmic mobilization of GLTSCR2 in response to ribosomal stresses enhances nuclear p53 upregulation
The results above indicate that GLTSCR2 affects the stability of p53 through direct interactions with p53. However, the majority of endogenous GLTSCR2 is located in the nucleolus, rather than in the nucleoplasm where p53 and MDM2 are located, whereas ectopic GLTSCR2 is detected in both the nucleolus and nucleoplasm (Figure 6a). This raises questions about how endogenous nucleolar GLTSCR2 forms a complex with nucleoplasmic p53 to inhibit p53 degradation. Therefore, we examined whether nucleolar GLTSCR2 was mobilized to the nucleoplasm under ribosomal stresses requiring p53 induction and cell cycle inhibition, such as hypoxia or actinomycin D (Act-D) treatment. After applying nucleolar stresses to A549 and H1299 cells, we observed that GLTSCR2 was diffusely redistributed throughout the nucleoplasm, irrespective of p53 status (Figure 6b). The change in distribution was not due to nucleolar disassembly caused by ribotoxic stresses because the nucleolar architecture was well preserved, as seen in differential interference contrast (DIC) images (Figure 6b). We then hypothesized that nucleoplasmic redistribution by ribosomal stresses may increase the opportunities for GLTSCR2–p53 interaction, thereby enhancing p53 upregulation. To test this hypothesis, A549-shGLT cells, in which GLTSCR2 expression was downregulated by shRNA-introducing lentivirus (Supplementary Figure S5a), were exposed to hypoxia or treated with Act-D. Then, nuclear p53 fluorescent intensity was determined after immunostaining with anti-p53 and anti-GLTSCR2 antibodies. When exposed to hypoxia, nuclear p53 intensity was increased 4.2-fold (shScr+H) and 3.7-fold (shGLT+H) in A549 cells with nucleoplasmic GLTSCR2 distributions compared with the levels in matched cells with nucleolar GLTSCR2 localization (shScr and shGLT, respectively; Figures 6c and d). When treated with Act-D, p53 intensity was increased 4.1-fold (shScr+A) and threefold (shGLT+A) compared with shScr and shGLT, respectively. Furthermore, profound increases in the intensity of nuclear p53 in shScr+H (2.1-fold increase) or shScr+A (4.4-fold increase) compared with that of shGLT+H or shGLT+A, respectively, support the importance of GLTSCR2 in p53 regulation under ribosomal stress. Consistent with these findings, GLTSCR2–p53 interaction was increased in shScr+H and shGLT+H cells compared with the levels seen in shScr and shGLT cells, respectively (Figure 6e, left panel). These findings were observed in cells treated with Act-D (Figure 6e, right panel). We obtained similar results from experiments using human keratinocytes (Supplementary Figure S6). Next, to demonstrate the ratio of GLTSCR2-unbound p53 to GLTSCR2-bound p53 in normal status or under ribosomal stress conditions, we performed immunoprecipitation using excess anti-GLTSCR2 antibody and western blot for p53 in the supernatant (unbound p53) and pellet (bound p53). Densitometric analysis demonstrated that 27% of total p53 was unbound to GLTSCR2 in unstressed conditions while 77 and 66% were bound in hypoxia and Act-D-treated conditions, respectively. Taken together, our data indicate that GLTSCR2 is translocated to the nucleoplasm in response to ribotoxic stress and that nuclear GLTSCR2 effectively enhances p53 upregulation.
Nucleoplasmic mobilization of GLTSCR2 by ribosomal stress increases GLTSCR2–p53 interaction. (a) A549 cells were co-immunostained with anti-GLTSCR2 and anti-nucleolin antibodies (Endo) or transfected with GFP-GLTSCR2 plasmid (Ecto), and viewed under a confocal microscope. (b) The indicated cells were untreated (control), treated with 50 ng/ml Act-D for 6 h, or exposed to 0.4% hypoxia for 12 h. The cells were co-immunostained with anti-GLTSCR2 and anti-nucleolin antibodies, and viewed with a DIC-equipped confocal microscope. (c) A549 cells were infected with Lenti-shGLT (shGLT) or Lenti-shScr (shScr) for 48 h. Then, cells were exposed to 0.4% hypoxia (+H) or maintained in normoxia for an additional 12 h, or incubated in the presence (+A) or absence of 50 ng/ml Act-D for 6 h. The cells were double immunostained with anti-GLTSCR2 and anti-p53 antibodies, and p53 fluorescence intensity was determined by confocal microscopy after nuclear staining with DAPI. The histogram shows the average p53 fluorescence intensity of at least 100 cells for each group. Data are presented as fold increase in intensity compared with control cells. *P<0.01 (d) Representative immunocytochemical images of (c). A, Act-D; H, hypoxia. (e) Cell lysates from (c) were immunoprecipitated with anti-GLTSCR2 antibody, followed by western blotting for p53 (upper panel). Western blots for input GLTSCR2 was shown in middle panels. Loadings were normalized to p53 (lower panel). (f) Cell lysates from (c) were immunoprecipitated with excess amounts of anti-GLTSCR2 antibody. After precipitation, supernatant (S) and precipitated pellets (P) were subjected to western blots for p53 and GLTSCR2 (left panel). The histogram shows the ratio of p53 in the supernatant to total p53 (right panel), determined by densitometric analysis of three independent experiments. Hyp, hypoxia; In., 20% input; U, untreated
Overexpression of GLTSCR2 suppresses tumor growth in a xenograft animal model
We performed xenograft animal experiments to investigate the role of GLTSCR2 in in vivo tumor growth. We constructed HT29-p53 cells by stably transfecting pcDNA-p53 to HT29 cells. HT29 and HT29-p53 cells infected by adenovirus expressing GLTSCR2 were injected into opposite flanks of nude mice, and the weights of tumor masses were compared after 3 weeks of inoculation. Ectopic expression of GLTSCR2 resulted in suppression of tumor growth in HT29-p53 cells compared with parent HT29 cells (Figures 7a and b). Expressions of GLTSCR2 and p53 in tumor masses formed by inoculations of HT29 and HT29-p53 cells are shown in Figure 7c. Our data show that GLTSCR2 expression suppresses in vivo tumor growth in the p53-dependent pathway.
GLTSCR2 inhibits tumor cell proliferation in a xenograft animal model. (a) HT29 and HT29-p53 cells infected by Ad-GLT/GFP were subcutaneously inoculated on the dorsal left or right flanks of 6-week-old nude mice (n=10), respectively. Tumor masses were excised 21 days after inoculation, and tumor mass weights (mean±S.D.) were compared. *P<0.05. (b) Representative pictures of tumor masses from five mice. The bar length is 1 cm. (c) Representative immunohistochemical images for GLTSCR2 and p53 expressions. Original magnification × 100
Discussion
The nucleolus is an organelle of the interphase cell nucleus where the biogenesis of ribosomes occurs. Thus, cell proliferation processes with high biosynthetic demands are closely coordinated with nucleolar function.28 In addition to ribosome biogenesis, accumulating evidence has demonstrated that the nucleolus directly controls the cell cycle and cell death in certain physiological and pathological states including cancers.29 Regulation of these functions by the nucleolus is accomplished by a meshwork of proteins encompassing the nucleolus and nucleoplasm. One of the crucial protein axes responsible for cell cycle and cell death regulation is the nucleolus-MDM2-p53 pathway, in which nucleolar proteins control the stability of p53 mainly through the inhibition of MDM2 activity.15, 22, 30 For example, ARF13 and NS12 are released from the nucleolus to the nucleoplasm, where they bind MDM2 to suppress MDM2-mediated ubiquitination and degradation of p53. NPM also participates in ARF-dependent or -independent regulatory mechanisms of p53 stability control.31, 32 These findings demonstrate the importance of nucleolar proteins in p53 regulation. Here, we identified GLTSCR2 as another member of the family of nucleolar proteins that regulate cellular p53 levels in response to ribosomal stress.
Results of immunoprecipitation, pull-down, and transfection assays of wild-type and mutant GLTSCR2 indicate that GLTSCR2 directly binds to p53 and that the interaction of GLTSCR2 with p53 is crucial for translational upregulation of its binding partner, p53. Previous reports showed that competition with MDM2 for p53 binding and the resulting interference with MDM2-dependent ubiquitination is the key molecular mechanism by which some nucleolar proteins regulate p53 stability.33 However, our results show that GLTSCR2 does not directly interact with MDM2 while GLTSCR2 stabilizes p53 in ARF-deleted A549 cells by inhibiting MDM2-mediated ubiquitination of p53. ARF is a major upstream activator of p53 and functions as a tumor suppressor against oncogenic stimulation.34 However, the ARF gene is commonly mutated, deleted, or silenced, and the frequency of ARF inactivation in human cancer is second only to the loss of p53.16 In cells in which ARF is inactivated by genetic or epigenetic events, the ARF-MDM2-p53 axis is disrupted and p53 is inactivated.35 Under conditions where the ARF-MDM2-p53 axis is dysregulated, GLTSCR2 might have a key role in p53 activation through an ARF-independent pathway. Therefore, we suggest that GLTSCR2 exerts cell cycle and cell death regulation through direct p53 activation in tumors lacking functional ARF.
Appropriate subcellular localization is crucial for the regulation of p53 function. Export of p53 from the nucleus to the cytoplasm is mediated by a nucleas export signal (NES; residues 340–351) located in its TTD.36 The masking and unmasking of NES within oligomers may be a general mechanism regulating the subcellular localization of p53. Stommel et al.36 demonstrated that p53 tetramerization occludes its NES, thereby leading to nuclear retention of the DNA-binding form. In contrast, the conversion of tetramers into monomers or dimers exposes the p53 NES to the proteins that mediate export to the cytoplasm. Our deletion mapping studies show that the TTD involving the p53 NES is important for GLTSCR2–p53 interaction. In addition, our oligomerization assay shows that formation of the tetramer form of p53 is increased upon GLTSCR2 overexpression. In this process, physical interactions between GLTSCR2 and p53 seem to be important because M3 and M4 GLTSCR2 mutants, which contain only one p53-binding motif, have low potential to form p53 tetramer. Our results suggest that GLTSCR2–p53 interaction is a crucial mechanism preventing MDM2-mediated degradation of p53. Contrary to our results, however, tetrameric p53 is thought to bind MDM2 with higher affinity than monomeric form and tetramerization is required for p53 to be efficiently ubiquitinated by MDM2.27, 37 These contradictory results may be explained in part by a previous report, indicating that the C-terminal TTD of p53 is also required for efficient MDM2 binding and ubiquitination, although the MDM2-binding site in p53 is located in the N-terminus of p53.38 It is possible that GLTSCR2 binding to the TTD of p53 assumes a more complex tertiary structure, which reduces the affinity to MDM2 otherwise increased by tetramerization.
Our results indicate that GLTSCR2 has a role in p53 stability control through direct interaction with p53. However, the majority of cellular GLTSCR2 is in the nucleolus, not in the nucleoplasm where p53 and MDM2 are located. This raises questions about how nucleolar GLTSCR2 forms a complex with p53. We showed that GLTSCR2 was redistributed from the nucleolus to the nucleoplasm in response to ribosomal stresses, such as hypoxia or Act-D, and the interaction of GLTSCR2 with p53 increased in parallel with the nucleoplasmic mobilization of GLTSCR2 (Figure 6). Together with our recent report that GLTSCR2 is translocated to the nucleoplasm in response to DNA damage,39 this finding indicates that GLTSCR2 is a dynamic protein that shuttles between the nucleolus and nucleoplasm, similar to other p53-regulating nucleolar proteins such as NPM and NS.8, 12
Nucleolar proteins interact with each other to form intricate networks for the regulation of p53. For instance, NPM directly interacts with p53 or nucleolar ARF to regulate MDM2-mediated p53 ubiquitination.15, 21, 22 NS, another nucleolar protein regulating MDM2 stability,12 interacts with NPM,40 forming a complicated meshwork of p53-regulating proteins. It is possible that GLTSCR2 may interact and function in concert with other nucleolar proteins involved in nucleolus-p53 regulatory axes. We are now studying the role of GLTSCR2 in NPM-MDM2-p53 axis.
Recently, Sasaki et al.41 reported that GLTSCR2 (also known as PICT1) decreases p53 stability through RPL11-dependent pathway. Using GLTSCR2-deficient ES cells, they showed that GLTSCR2 binds RPL11. In the absence of GLTSCR2, RPL11 is released from the nucleolus to block the MDM2-mediated ubiquitination of p53, thereby inhibiting cell cycle progression. The findings of Sasaki et al. contradict our findings. However, the NS-MDM2 axis model of p53 regulation can explain the discrepancies between study results, at least in part. NS has a dual role in p53 regulation: both up and downregulation of NS activate the p53 pathway to inhibit cell cycle progression. Depletion of NS induced the interactions of RPL5 and RPL11 with MDM2, which suppress MDM2-mediated p53 degradation.42, 43 This mechanism is similar to that observed in GLTSCR2-deficient cells. Paradoxically, aberrant overexpression of NS also prevents p53 degradation through the inhibition of MDM2-mediated p53 ubiquitination by the physical interaction of NS with MDM2.43 The nucleoplasmic release of GLTSCR2 and interactions of GLTSCR2 and p53 may have a crucial role in p53 regulation under physiological conditions or GLTSCR2 overexpression. In conditions of GLTSCR2 deficiency, however, the p53 regulatory mechanism of GLTSCR2 through nucleoplasmic mobilization is not activated, which may induce the activation of alternative pathways such as RPL11-mediated suppression of MDM2 to maintain p53 stability, as shown by Sasaki et al.41 Thus, the release of RPL11 from the nucleolus and interaction with MDM2 may be a major mechanism of p53 activation in GLTSCR2 deficiency. These discrepant results concerning GLTSCR2 imply that tight regulation of GLTSCR2 expression level is important for cellular homeostasis, and that stresses inducing perturbations of cellular GLTSCR2 levels activate p53.
In summary, we show that GLTSCR2 translationally upregulates p53 by increasing p53 stability. After exposure to ribosomal stresses, such as hypoxia and Act-D, GLTSCR2 is mobilized from the nucleoli to the nucleoplasm leading to enhanced formation of the p53–GLTSCR2 complex, which interferes with the degradation of p53 by inhibiting MDM2-mediated ubiquitination of p53. This GLTSCR2-p53 regulatory axis functions without the involvement of ARF, a major p53-upstream activator. Therefore, our data indicate that GLTSCR2 is a key upstream regulator of p53 in cancers in which ARF is inactivated by genetic or epigenetic events. Furthermore, GLTSCR2 could be a therapeutic target for the induction of cancer cell death in cases with inactivated nucleolus-ARF-p53 axis.
Materials and Methods
Cell culture, plasmid construction, and transfection
Cells were cultured in appropriate media supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics: H1299, HCT116, HT29, and HeLa in RPMI; A549 in DMEM; human epidermal keratinocytes (Invitrogen, Gibco Cell Culture, Portland, OR, USA) in Epilife basal medium supplemented with Epilife defined growth supplement (Invitrogen). Plasmids for wild-type or mutant forms of GLTSCR2 and p53 were generated using PCR and standard cloning techniques as described previously.20 Cells were transfected with plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. HT29-p53 cells stably expressing p53 were generated by transfecting HT29 cells with a p53-expressing plasmid (pcDNA-p53) and selection with G418.
Antibodies and reagents
Anti-GLTSCR2 rabbit polyclonal antibody was purified from immunized antiserum as described previously.18 Anti-p53 mouse monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used for immunoprecipitation and western blotting. Anti-p21, anti-MDM2, anti-GFP, anti-V5, and anti-NPM antibodies were purchased from Abcam (Cambridge, UK). Anti-p16, anti-p27, and anti-phospho-p53 antibodies were obtained from Cell Signaling Technology (Boston, MA, USA). Anti-α tubulin and anti-HA antibodies were from Santa Cruz Biotechnology, Inc. and Roche Applied Science (Burgess Hill, UK), respectively. Unless otherwise specified, all other reagents were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
Construction of the Tet-off adenoviral-mediated system
The assembly and production of recombinant adenovirus was performed according to the manufacturer’s instructions (Adeno-X™ Tet-Off Expression System 1, Clontech Laboratories, Inc., Mountain View, CA, USA). A GFP-GLTSCR2 construct consisting of full-length GLTSCR2 cDNA fused to a C-terminal GFP tag was first cloned into pTRE-Shuttle2 vector (Clontech), which contains the tetracycline-responsive element (TRE) upstream of the CMV minimal promoter. The resulting TRE-GFP-GLTSCR2 expression unit was excised from the pTRE-Shuttle2 vector using I-Ceu I and PI-Sce I resection enzymes, and then ligated to Swa I-digested Adeno-X System 1 Viral DNA (Clontech). The resulting recombinant Adeno-X-GFP-GLTSCR2 vector (Ad-GFP/GLT) was packaged into infectious adenoviral particles by transfection of HEK293 cells, and recombinant adenovirus was harvested by lysing transfected cells. To transiently express GLTSCR2, cells were co-infected with recombinant adenovirus (Ad-GFP/GLT) and regulation virus (Adeno-X Tet-Off Virus) and incubated in serum-free medium for 12 h, before changing to a fresh complete medium.
Knockdown of GLTSCR2 and lentiviral infection
GLTSCR2 knockdown in A549 and H1299 cells were established using a Lentivector-based shRNA system according to the manufacturer’s instructions (GeneCopoeia Inc., Rockville, MD, USA). The GLTSCR2-targeted sequence was 5′-GAG ACC GGT TCA AGA GCT T-3′, and the scrambled shRNA sequence was 5′-CGA TAC TGA ACG AAT C-3′. For lentiviral-mediated gene transfer, these sequences were cloned into the psiLv-H1 shRNA expression vector and transfected into HEK293 cells for pseudoviral packaging. Supernatants were collected and purified viral particles were titered and used to infect cells.
Western blotting, immunofluorescence, and immunoprecipitation
Western blotting was performed as described previously.20 For immunostaining, pretreated cells were fixed with 4% paraformaldehyde and incubated sequentially with primary antibody overnight at 4 °C and secondary antibody for 2 h at 4 °C. Cells were viewed under a Nomarski DIC-equipped inverted confocal microscope (META 510, Zeiss, Jena, Germany) after nuclear staining with DAPI. For immunoprecipitation, cells were lysed in lysis buffer (500 mM NaCl, 50 mM Tris-HCl pH 7.5, 0.5% Triton X-100, 1 mM EDTA, and 1 μM DTT), clarified by centrifugation, incubated with the indicated antibodies, and immunoprecipitated with protein A (Sigma-Aldrich). The precipitates were washed four times, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed by western blotting.
Flow cytometry
A549 cells infected by Ad-GFP/GLT or Ad-GFP were fixed with cold 70% ethanol for 3 h, treated with RNase (20 μg/ml) at 4 °C for 15 min, and finally stained with 50 μg/ml propidium iodide in PBS at room temperature for 20 min. The samples were analyzed in a Cytomics FC500 flow cytometer (Beckman Coulter, Kraemer Blvd, CA, USA) and the results were analyzed using the CXP software (Beckman Coulter).
Oligomerization and ubiquitination assay
For the detection of p53 oligomers, A549 cells were transfected with indicated plasmids for 24 h and harvested. Cell lysates were treated with glutaraldehyde at a final concentration of 0.05% at room temperature for 20 min and then analyzed by western blotting as described previously.21 Ubiquitination assay was performed using the Ubiquitin Enrichment Kit (Pierce, Rockford, IL, USA) according to the manufacturer’s protocol.
Semiquantitative RT-PCR analysis and ChIP assay
We converted 1 μg of total RNA to cDNA. The RT-PCR exponential phase was determined on 22–32 cycles to allow semiquantitative comparisons. The PCR regimen for p53 involved an initial denaturation step of 94 °C for 5 min, followed by 32 cycles at 94 °C for 30 s, 56 °C for 45 s, and 72 °C for 45 s on a GeneAmp PCR system 9700 (Perkin-Elmer, Santa Clara, CA, USA). The sequences of the gene-specific primers are as follows: p53 forward primer 5′-ATCAAGCGAGCACTGCCCAA-3′, reverse primer 5′-TTATGGCGGGAGGTAGACTG-3′; GAPDH forward primer 5′-AACCATGAGAAGTATGACAA-3′, reverse primer 5′-CTGCTTCACCACCTTCTTGA-3′. The ChIP assay was performed using a chip assay kit (Upstate, Lake Placid, NY, USA) as recommended by the manufacturer. The precipitated DNA was analyzed by quantitative PCR using the following specific primers. Primers for p21 promoter were 5′-GCTGTGGCTCTGATTGGCTTT-3′ (forward) and 5′-ACAGGCAGCCCAAGGACAAA-3′ (reverse); primers for p21 non-specific site were 5′-GAGTCCTGTTTGCTTCTGGGC-3′ (forward) and 5′-CTGCATTGGGGCTGCCTATGT-3′ (reverse); primers for GAPDH promoter were 5′-GGACTCATGACCACAGTCCAT-3′ (forward) and 5′-GTTCAGCTCAGGGATGACCTT-3′ (reverse).
In vivo tumor propagation and immunohistochemistry
Experiments using mice were performed in accordance with the guidelines of the Ethical Committee of the Kyung Hee University (KHP-2011-01-4). For transplantation of cancer cells, 3 × 107 cells were suspended in 200 μl of RPMI with 10% FBS and were injected subcutaneously into nude mice (BALB/c-nu slc). After 2 weeks, visible tumors were observed and were measured every 3 days for 3 weeks and then analyzed after extraction. Expression of GLTSCR2 and p53 was detected from formalin-fixed tumor tissue by conventional immunohistochemistry using anti-GLTSCR2 or p53 antibody, as described previously.44
Statistics
Statistical analyses were performed using the SPSS software, version 12.0 (SPSS, Chicago, IL, USA). Data were analyzed with two-tailed, unpaired Student’s t-tests or ANOVA. Differences were considered statistically significant when P<0.05.
Abbreviations
- ARF:
-
alternative reading frame
- GFP:
-
green fluorescence protein
- GLTSCR2 :
-
glioma tumor-suppressor candidate region gene 2
- GST:
-
glutathione S-transferase
- MDM2:
-
murine double minute 2
- NES:
-
nucleus export signal
- NLS:
-
nuclear localization signal
- NPM:
-
nucleophosmin
- NS:
-
nucleostemin
- RPL5:
-
ribosomal protein L5
References
Vogelstein B, Lane D, Levine AJ . Surfing the p53 network. Nature 2000; 16: 307–310.
Oren M . Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10: 431–442.
Hollstein M, Sidransky D, Vogelstein B, Harris CC . p53 mutations in human cancers. Science 1991; 5: 49–53.
Haupt Y, Maya R, Kazaz A, Oren M . Mdm2 promotes the rapid degradation of p53. Nature 1997; 15: 296–299.
Kubbutat MH, Jones SN, Vousden KH . Regulation of p53 stability by Mdm2. Nature 1997; 15: 299–303.
Michael D, Oren M . The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol 2003; 13: 49–58.
Zhang Y, Lu H . Signaling to p53: ribosomal proteins find their way. Cancer Cell 2009; 6: 369–377.
Brady SN, Yu Y, Maggi LB, Weber JD . ARF impedes NPM/B23 shuttling in an Mdm2-sensitive tumor suppressor pathway. Mol Cell Biol 2004; 24: 9327–9338.
Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH . Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell 2003; 3: 577–587.
Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H . Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol 2004; 24: 7654–7668.
Dai MS, Lu H . Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J Biol Chem 2004; 279: 44475–44482.
Meng L, Lin T, Tsai RY . Nucleoplasmic mobilization of nucleostemin stabilizes MDM2 and promotes G2-M progression and cell survival. J Cell Sci 2008; 15: 4037–4046.
Sherr CJ . The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol 2001; 2: 731–737.
Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L et al. The INK4a tumor suppressor gene product, p19ARF, interacts with mdm2 and neutralizes mdm2’s inhibition of p53. Cell 1998; 92: 713–723.
Zhang Y, Xiong Y, Yarbrough WG . ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 1998; 92: 725–734.
Saporita AJ, Maggi LB, Apicelli AJ, Weber JD . Therapeutic targets in the ARF tumor suppressor pathway. Curr Med Chem 2007; 14: 1815–1827.
Okahara F, Itoh K, Nakagawara A, Murakami M, Kanaho Y, Maehama T . Critical role of PICT-1, a tumor suppressor candidate, in phosphatidylinositol 3,4,5-trisphosphate signals and tumorigenic transformation. Mol Biol Cell 2006; 17: 4888–4895.
Kim Y-J, Cho Y-E, Kim Y-W, Kim J-Y, Lee S, Park J-H . Suppression of putative tumour suppressor gene GLTSCR2 expression in human glioblastomas. J Pathol 2008; 216: 218–224.
Okahara F, Ikawa H, Kanaho Y, Maehama T . Regulation of PTEN phosphorylation and stability by a tumor suppressor candidate protein. J Biol Chem 2004; 29: 45300–45303.
Yim J-H, Kim Y-J, Ko J-H, Cho Y-E, Kim S-M, Kim J-Y et al. The putative tumor suppressor gene GLTSCR2 induces PTEN-modulated cell death. Cell Death Differ 2007; 14: 1872–1879.
Colombo E, Marine JC, Danovi D, Falini B, Pelicci PG . Nucleophosmin regulates the stability and transcriptional activity of p53. Nat Cell Biol 2002; 4: 529–533.
Dai MS, Sun XX, Lu H . Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol Cell Biol 2008; 28: 4365–4376.
Sakaguchi K, Sakamoto H, Lewis MS, Anderson CW, Erickson JW, Appella E et al. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry 1997; 19: 10117–10124.
Foo RS, Nam YJ, Ostreicher MJ, Metzl MD, Whelan RS, Peng CR et al. Regulation of p53 tetramerization and nuclear export by ARC. Proc Natl. Acad Sci USA 2007; 26: 20826–20831.
McLure KG, Lee PW . How p53 binds DNA as a tetramer. EMBO J 1998; 17: 3342–3350.
Shieh SY, Taya Y, Prives C . DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. EMBO J 1999; 18: 1815–1823.
Maki CG . Oligomerization is required for p53 to be efficiently ubiquitinated by MDM2. J Biol Chem 1999; 274: 16531–16535.
Montanaro L, Treré D, Derenzini M . Nucleolus, ribosomes, and cancer. Am J Pathol 2008; 173: 301–310.
Emmott E, Hiscox JA . Nucleolar targeting: the hub of the matter. EMBO Rep 2009; 10: 231–238.
Kurki S, Peltonen K, Latonen L, Kiviharju TM, Ojala PM, Meek D et al. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell 2004; 5: 465–475.
Bertwistle D, Sugimoto M, Sherr CJ . Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol Cell Biol 2004; 24: 985–996.
Lambert B, Buckle M . Characterisation of the interface between nucleophosmin (NPM) and p53: potential role in p53 stabilisation. FEBS Lett 2006; 9: 345–350.
Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ . Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci USA 1998; 95: 8292–8297.
Weber JH, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D . Nucleolar ARF sequesters Mdm2 and activates p53. Nat Cell Biol 1999; 1: 20–26.
Serrano M . The INK4a/ARF locus in murine tumorigenesis. Carcinogenesis 2000; 21: 865–869.
Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM . A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J 1999; 18: 1660–1672.
Marston NJ, Jenkins JR, Vousden KH . Oligomerisation of full length p53 contributes to the interaction with mdm2 but not HPV E6. Oncogene 1995; 10: 1709–1715.
Chen J, Marechal V, Levine AJ . Mapping of the p53 and mdm-2 interaction domains. Mol Cell Biol 1993; 13: 4107–4114.
Kim JY, Seok KO, Kim YJ, Bae WK, Lee S, Park JH . Involvement of GLTSCR2 in the DNA damage response. Am J Pathol 2011; 179: 1257–1264.
Ma H, Pederson T . Nucleophosmin is a binding partner of nucleostemin in human osteosarcoma cells. Mol Biol Cell 2008; 19: 2870–2875.
Sasaki M, Kawahara K, Nishio M et al. Regulation of the MDM2-P53 pathway and tumor growth by PICT1 via nucleolar RPL11. Nat Med 2011; 17: 944–951.
Ma H, Pederson T . Depletion of the nucleolar protein nucleostemin causes G1 cell cycle arrest via the p53 pathway. Mol Biol Cell 2007; 18: 2630–2635.
Dai MS, Sun XX, Lu H . Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol Cell Biol 2008; 28: 4365–4376.
Kim JY, Kim HS, Lee S, Park JH . The expression of GLTSCR2, a candidate tumor suppressor, is reduced in seborrheic keratosis compared to normal skin. Pathol Res Pract 2010; 206: 295–299.
Acknowledgements
This work was supported by the National Research Foundation of Korea (KRF) grant funded by the Korea government (MEST; no. 2011-0030725 and 2010-0010156 to SL). This work was performed during the sabbatical year (2011) of S Lee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by M Oren
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Rights and permissions
About this article
Cite this article
Lee, S., Kim, JY., Kim, YJ. et al. Nucleolar protein GLTSCR2 stabilizes p53 in response to ribosomal stresses. Cell Death Differ 19, 1613–1622 (2012). https://doi.org/10.1038/cdd.2012.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2012.40
Keywords
This article is cited by
-
Changes in calpain-2 expression during glioblastoma progression predisposes tumor cells to temozolomide resistance by minimizing DNA damage and p53-dependent apoptosis
Cancer Cell International (2023)
-
Microglial cannabinoid receptor type 1 mediates social memory deficits in mice produced by adolescent THC exposure and 16p11.2 duplication
Nature Communications (2023)
-
Nucleolar protein NOC4L inhibits tumorigenesis and progression by attenuating SIRT1-mediated p53 deacetylation
Oncogene (2022)
-
Gene expression signatures of individual ductal carcinoma in situ lesions identify processes and biomarkers associated with progression towards invasive ductal carcinoma
Nature Communications (2022)
-
NOP53 undergoes liquid-liquid phase separation and promotes tumor radio-resistance
Cell Death Discovery (2022)