Abstract
The number of Drosophila egg chambers is controlled by the nutritional status of the female. There is a developmental checkpoint at stage 8, which is controlled by BR-C in the follicle cells along with ecdysteroid. During this period, developmental decision is made in each egg chamber to determine if it will develop or die. During nutritional shortage, inducing apoptosis in the nurse cells of stages 8 and 9 egg chambers reduces the number of egg chambers. We show that ecdysone response genes E75A and E75B are involved in inducing or suppressing apoptosis. It is thus possible that the E75 isoforms A and B are involved in the decision to develop or die in oogenesis. We have established part of the pathway by which ecdysone response genes control apoptosis of the nurse cells and hence select between degeneration or development of individual egg chambers at stages 8 and 9.
Similar content being viewed by others
Introduction
The Drosophila egg chamber consists of a cluster of 16 cells interconnected by ring canals, which are surrounded by a monolayer of somatic follicle cells. One cell in the cluster develops into the oocyte and the other 15 cells become nurse cells that support the oocyte in its development.1 The 15 nurse cells degenerate by apoptosis when the oocyte is mature.2, 3 Normally, apoptosis of the nurse cells is completed at stage 14 of oogenesis when they have transferred their contents to the oocyte.3, 4 Apoptosis of the nurse cells commences at stage 10B, when nurse cells undergo cytoskeletal rearrangement.5, 6 However, in flies that are maintained under starvation conditions or have 20-hydroxyecdysone (20E) at physiological concentrations injected into the abdomen, apoptosis is observed in the nurse cells much earlier, at stages 8 and 9.7, 8 Simultaneous application of the juvenile hormone analogue (JHA), Methoprene, inhibits this apoptosis.7, 8 The ecdysteroid concentration in starved females is higher than in females that are maintained on normal food.9 Thus it seems likely that the monitoring of nutrition and the response of the fly in terms of oogenesis will be controlled in part by ecdysone and juvenile hormone (JH).
Ecdysone functions through the Ecdysone/Ultraspiracle nuclear receptor complex (EcR/USP).10, 11 The complex directly regulates the early ecdysone response genes such as the Broad-Complex (BR-C), E74 and E75. The BR-C encodes a family of zinc finger transcription factors.12 E75 encodes three orphan members of the nuclear receptor superfamily, designated E75A, E75B and E75C.13 Early ecdysone response genes are essential for oogenesis. Transcription of E75 and E74 appeared to be upregulated during stage 8 in both the nurse cells and somatic follicle cells.14 Germline clones of E75 mutant cells result in degeneration of egg chambers after stage 8, with a phenotype similar to that of the temperature-sensitive mutant ecdysoneless1.14 The ecdysone receptor, EcR, which is crucial for regulation of expression of the early response genes, has also been shown to be crucial in the germline, as mutant germline clones arrest development during mid-oogenesis.14 In addition, we have shown that EcR isoforms are differentially suppressed, with EcRA showing higher expression and EcRB lower expression under nutritional shortage.15 BR-C expression in the somatic follicle cells regulates the establishment of dorso-ventral polarity of the eggshell later in oogenesis16 and leads to prolonged endoreplication and to additional amplification of selected genes.17 In addition, BR-C expression in the follicle cells controls the cell fate of the egg chamber, determining if it will progress to develop into an egg or induce apoptosis.8 BR-C isoforms, Z1 and Z3, affect yolk protein gene expression, while Z2 and Z3 expression in the follicle cells induce apoptosis of the stages 8 and 9 egg chambers.8 Thus ecdysone, ecdysone receptor and ecdysone response genes are clearly crucial for a number of developmental decisions in normal oogenesis.
Apoptosis in many tissues and glands in insects is induced by 20E and is regulated by the same ecdysone response genes that are crucial in oogenesis.18 Dronc is one of the apoptosis inducers in Drosophila,19 and is upregulated by reaper,19, 20 which also induce apoptosis in Drosophila.21, 22 Reaper expression is regulated by BR-C and hid expression is regulated by BR-C and E74A in Drosophila salivary glands.23 But Foley and Cooley4 have established that reaper, grim and hid do not affect the apoptosis of nurse cells that commences during normal development of an egg at stage 12 of oogenesis.4 E75 is required for the suppression of diap2, which inhibits the progression of apoptosis in the larval salivary glands during Drosophila metamorphosis.23, 24 The relationship between BR-C, E75A and E75B is described by Woodard et al.25 and White et al.26 during early metamorphosis in Drosophila. The ecdysone/EcR/USP complex activates a small set of genes (early and early-late genes), some of which encode transcription factors. These gene products in turn negatively autoregulate, and turn on a large set of effecter genes (late genes), and control the expression of the mid-prepupal factor βFTZ-F1. This factor then specifies the appropriate early gene response to the subsequent prepupal pulse of ecdysone.25, 26
In this paper, we describe the network of ecdysone response genes used in the control of apoptosis of nurse cells in the stages 8 and 9 egg chamber of Drosophila. BR-C Z2 and Z3, along with E75A and E75B in the follicle cells, control the premature apoptosis of the nurse cells. BR-C responds to the signal initiated by nutritional or hormonal conditions, and controls the expression of E75A and B in the egg chamber. E75A and B have opposite effects on apoptosis, E75A acts as an apoptosis inducer and E75B acts as an apoptosis inhibitor.
Results
E75A expression in the ovary
Ecdysone response genes have been shown to be involved in developmental decisions in many insect tissues and glands.18 We investigated the early ecdysone response genes, E75A and E75B, to establish whether or not they affect apoptosis in the nurse cells of the egg chamber at stages 8 and 9. Transgenic flies of E75A and E75B were heat shocked to induce overexpression in the egg chamber, both in the nurse cells and follicle cells. E75A overexpression induced 12- and 15-fold higher expression than in OrR under fed and starved conditions, respectively, and E75B overexpression induced 19- and 17-fold higher expression than in OrR under fed and starved conditions, respectively. E75A overexpression in the egg chamber induced apoptosis of the nurse cells at stages 8 and 9 in fed flies (F3) and the percentage of egg chambers undergoing apoptosis increased in starved flies (F3S1). E75B overexpression in the egg chamber suppressed the apoptosis of nurse cells at stages 8 and 9 in starved flies (Table 1). E75A and E75B were then investigated to see if they showed a different temporal or spatial expression pattern in the ovary when comparing apoptotic (starved and 20E injected) and nonapoptotic (fed and JH-treated) conditions.
E75A expression was observed in the germarium, and follicle cells and nurse cells at early stages, then in the nurse cells at stage 10 of oogenesis in fed flies. However, in the egg chambers at stages 8 and 9, E75A expression in the follicle cells was not observed and expression in the nurse cells was weak (Figure 1A a and f). On the other hand, E75A expression at stages 8 and 9 was observed in follicle cells of starved (S3 and F3S1) flies (Figure 1A b and g) and was also present in the follicle cells at stages 8 and 9 when 20E was injected into the abdomen of the fed flies (Figure 1A d and e). There was no obvious difference in E75A comparing levels in nurse cells between apoptotic and nonapoptotic conditions, but in the follicle cells E75A expression was significantly different (Figure 1A f and g). The expression of E75A at stages 8 and 9 in starved flies was suppressed by JHA, Methoprene-treatment of the abdomen (Figure 1A c). We carried out RT-PCR to compare E75A expression levels between apoptotic and nonapoptotic conditions, using total RNA from dissected egg chambers at stages 8 and 9 only (Figure 1B). In starved flies (S3 and F3S1), E75A expression levels at stages 8 and 9 were higher than in fed flies (F3), and JHA-application suppressed the higher levels in starved flies (F3JHS1). 20E inject ion of fed flies induced higher E75A expression at stages 8 and 9 (F3EF1).
E75A expression in ovaries of Drosophila under various nutritional and hormonal conditions and expression levels in stage 8 and 9 egg chambers. (A) Results of in situ hybridization to the ovaries of females under various hormonal or nutritional conditions are shown. (a) Fed: maintained with yeast for 3 days (F3), (b) starved: starved for 1 day after maintaining with yeast for 3 days (F3S1), (c: JHA treated: starved for 1 day with JHA application to the abodomen (1 μg/100 nl acetone) after maintaining with yeast for 3 days (F3JHS1), (d) and (e) 20E treated: fed for 1 day with 20E injection into the abdomen (100 pg/50 nl Ringer's solution) after maintaining with yeast for 3 days. (f) Fed and (g) starved are enlarged to show the egg chamber at stage 8 and focus on the follicle cells of fed (F3) and starved (F3S1) flies, respectively. The number on the panel indicates the stage of oogenesis. (B)Results of RT-PCR using total RNA from the egg chamber (n=150 flies) at stages 8 and 9 only are shown. F3: with yeast for 3 days, S3: without yeast for 3 days, F3S1: starved for 1 day after maintaining with yeast for 3 days, F3AS1: starved for 1 day with acetone application (100 nl) after maintaining with yeast for 3 days, F3JHS1: starved for 1 day with JHA application (1 μg/100 nl) after maintaining with yeast for 3 days, F3RF1: fed for 1 day with Ringer's injection (50 nl) after maintaining with yeast for 3 days, F3EF1: fed for 1 day with 20E injection (100 pg/50 nl) after maintaining with yeast for 3 days. rp49 is used as a control. (C) Percentage of the egg chambers at stage 8 in which E75A expression is induced and at stage 9 in which apoptosis is induced in the nurse cells. Percentage of egg chamber expression was calculated as follows: {(number of egg chambers at stage 8 showing E75A expression in the follicle cells)/(total number of the egg chambers at stage 8)−(number of stage 8 egg chambers showing nuclear condensation or fragmentation in the nurse cells)} × 100. The percentage of egg chambers showing apoptosis in nurse cells at stage 9 was calculated as follows: {(number of egg chambers at stage 9, showing nuclear condensation and fragmentation in nurse cells)/(total number of egg chambers at stage 9)} × 100. Nuclear condensation and fragmentation were detected by staining with Hoechst. There were no significant differences between the percentage of egg chambers showing E75A expression in the follicle cells at stage 8 and the percentage showing apoptosis in the nurse cells at stage 9. *Indicates that there are significant differences (within 5%), between F3 versus S3 and F3S1, F3AS1 versus F3JHS1 and F3RF1 versus F3EF1. n=12 flies
Apoptosis of nurse cells following starvation and 20E injection was observed at late stage 8 or stage 9. E75A expression, however, was observed at stages 6 and 7 in all egg chambers of the females under all experimental conditions. If E75A expression induces apoptosis of the nurse cells at stages 8 and 9, the expression level in the follicle cells at stage 8 was likely to be important in determining whether or not apoptosis was induced. As shown in Figure 1C, there were no significant differences between the percentage of egg chambers at stage 8 in which E75A expression in the follicle cells was observed and the percentage of the nurse cells at stage 9 in which apoptosis was induced. This was true for all experimental conditions. But when compared with the controls, fed versus starved, starved versus JHA treated and fed versus 20E injected, these percentages changed dramatically (Figure 1C). The proportion of egg chambers expressing E75A at stage 8 and undergoing apoptosis at stage 9 was higher in starved flies (S3 and F3S1) than in fed flies (F3); and this higher level in starved flies was suppressed by JHA treatment. 20E treatment increased the proportions in fed flies.
We therefore propose that E75A expression in the follicle cells at stages 8 and 9 has a role in the induction of apoptosis in the nurse cells at stages 8 and 9. In flies, under nonapoptotic conditions, it seems that E75A expression level in the follicle cells is suppressed to prevent induction of apoptosis in the nurse cells at stages 8 and 9. E75A expression levels in the egg chamber are increased under apoptotic conditions, which leads to apoptosis in the nurse cells at stages 8 and 9.
E75B expression in the ovary
E75B expression was seen in region 2B of the germarium and the expression was faint in egg chambers at early stages. E75B expression was seen in the follicle cells and nurse cells at stages 4 or 5 again and expression continued until stage 9 in the ovary of fed flies (Figure 2A a and e). The E75B expression pattern was similar under all experimental conditions. However, expression levels appeared to be different by observation under the microscope when comparing nonapoptotic, fed and JHA treated with apoptotic, starved and 20E treated, flies. E75B overexpression in the egg chamber (in both nurse cells and follicle cells) suppressed apoptosis at stages 8 and 9 in starved flies (Table 1). E75B expression at stage 8 was observed in follicle cells and nurse cells (Figure 2A e, f, g and h), but expression levels were different in the egg chamber at stages 8 and 9 when comparing fed flies (F3) and starved flies (S3 and F3S1, Figure 2B and C). E75B expression levels in the egg chamber at stages 8 and 9 of starved flies were rescued by JHA treatment of starved flies (F3JHS1, Figure 2B and C). E75B is one of the ecdysone response genes in Drosophila, but E75B expression in the egg chamber at stages 8 and 9 was suppressed by 20E injection of fed flies (F3EF1, Figure 2B and C).
E75B expression in ovaries of Drosophila under various nutritional and hormonal conditions. (A)Results of in situ hybridization to the ovaries of females under various hormonal or nutritional conditions are shown. (a) Fed: maintained with yeast for 3 days (F3), (b) starved: starved for 1 day after maintaining with yeast 3 days (F3S1), (c) JHA treated: starved for 1 day with JHA application to the abdomen (1 μg/100 nl acetone) after maintaining with yeast for 3 days (F3JHS1), (d) 20E treated: fed for 1 day with 20E injection (100 pg/50 nl Ringer's solution) after maintaining with yeast 3 days. (e) Fed, (f) starved, (g) JHA treated and (h) 20E treated are enlarged the egg chamber at stage 8 to focus on the follicle cells of fed (F3), starved (F3S1), JHA treated (F3JHS1) and 20E treated (F3EF1) flies, respectively. The number on the panel indicates the stage of oogenesis. (B) Results of RT-PCR, using total RNA extracted from egg chambers (n=150 flies) at stage 8 and 9 only are shown. F3: with yeast for 3 days, S3: without yeast for 3 days, F3S1: starved for 1 day after maintaining with yeast for 3 days, F3AS1: starved for 1 day with acetone application (100 nl) after maintaining with yeast for 3 days, F3JHS1: starved for 1 day with JHA application (1 μg/100 nl) after maintaining with yeast for 3 days, F3RF1: fed for 1 day with Ringer's injection (50 nl) after maintaining with yeast for 3 days, F3EF1: fed for 1 day with 20E injection (100 pg/50 nl) after maintaining with yeast for 3 days. rp49 is used as a control. (C) The graph indicates the expression level using RT-PCR results that are shown in (B) using relative value±S.D. (measured by NIH image). *Indicates that there are significant differences between fed (F3) and starved (S3 and F3S1), starved (F3S1 and F3AS1) and JHA treated (F3JHS1) and fed (F3 and F3RF1) and 20E treated (F3EF1) flies
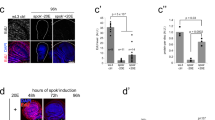
E75A and C mutants show similar lethal phases, but E75B mutants are viable and fertile, with no detectable phenotype.27 We thought that perhaps E75B mutants might be sensitive to nutritional stress even though under normal conditions they are fertile. Therefore, we examined E75B mutant flies that were maintained under nutritional shortage and checked how many stages 8, 9 and 10 egg chambers were present (Figure 3). The egg chambers of wild-type starved females were reduced by approximately 40, 65 and 70% of stages for 8, 9 and 10 egg chambers, respectively, compared to wild-type fed females, but the egg chambers of E75B-mutant starved flies was reduced approximately 65, 80% and over 80% for stages 8, 9 and 10 egg chambers, respectively (Figure 3a). JHA treatment of the starved wild-type flies suppresses apoptosis in stages 8 and 9 egg chambers7, 8 and led to recovery of the number of stages 8, 9 and 10 egg chambers (Figure 3b). However, when JHA was applied to the starved E75B mutant flies, the recovery levels were lower than in starved wild-type flies (Figure 3b). These results indicate that the E75B mutant is particularly sensitive to nutritional stress that E75B overexpression and JHA treatment suppressed this apoptosis at stages 8 and 9 (Table 1) and that JHA suppresses the apoptosis through E75B. From these results, we propose that E75A and E75B have opposite roles in determining if apoptosis of nurse cells at stages 8 and 9 is induced or not in Drosophila. Further, although E75B mutants are fertile and visible under normal conditions, this mutation is detrimental to fertility under conditions of nutritional stress.
E75B mutant is sensitive to starvation. We made E75B mutants (E75Δ1/E75Δ51) by crossing E75Δ1/TM6B, which is an ∼3 kb deletion, most of the first exon of E75B and E75Δ51/TM6B, which is an ∼30 kb deletion that removes the first exon of E75B, as well as the adjacent exon, shared by all three E75 isoforms, that encodes the second zinc finger of the DNA binding domain (Bialecki et al., 2002). (a) Percentage reduction of stages 8, 9 and 10 egg chambers comparing fed (F3) and starved (F3S1) flies is shown. n=12 flies. The egg chambers that indicated nurse cell apoptosis were not included in these graphs. (b) The percentage of recovery at stages 8, 9 and 10 egg chambers following JHA treatment of the starved flies (F3JHS1) is shown. n=12 flies. The egg chambers that showed nurse cell apoptosis were not included in these graphs
BR-C Z2 and Z3 control E75A and E75B expression in egg chambers
BR-C Z2 and Z3 are not expressed in the egg chambers at stages 8 and 9 of fed females, but they are expressed in the follicle cells under apoptotic conditions. In addition, overexpression in the egg chamber induces apoptosis of that egg chamber at stages 8 and 9 in fed flies.8 We have suggested that BR-C controls the developmental checkpoint at stages 8 and 9 of oogenesis in Drosophila,28 inducing either yolk protein synthesis or inducing apoptosis in the nurse cells at stages 8 and 9.8 On the basis of these results, we investigated whether or not Z2 and Z3 overexpression in the follicle cells affects E75A and E75B expression in egg chambers at stages 8 and 9.
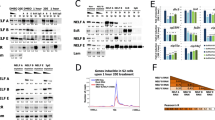
Figure 4A shows the E75A expression pattern in hsZ2 and Z3 transgenic flies under fed conditions (F3) with or without heat shock. E75A expression in the follicle cells at stages 8 and 9 was not seen or was very faint in hsZ2 and hsZ3 transgenic flies under fed conditions without heat shock (Figure 4A c, f and g). When Z2 and Z3 overexpression in the egg chamber was induced by heat shock, E75A was expressed in the egg chamber, in nurse cells and follicle cells at stages 8 and 9 under fed conditions (Figure 4A a, b, d and e). The E75A expression pattern in egg chambers at stages 8 and 9 thus reflects the results of in situ hybridization, as shown in Figure 3a (Figure 4B). E75A expression level at stages 8 and 9 following Z2 and Z3 overexpression was higher than the expression levels without heat-shock induction in not only fed flies but also starved flies (F3S1, Figure 4B). The percentage of egg chambers at stage 8 in which E75A expression was seen corresponded to the percentage of egg chambers at stage 9 in which apoptosis was induced in fed flies (Figure 4C). Z2 and Z3 overexpression increased the percentage of egg chambers at stage 8 in which E75A expression was observed and the percentage of nurse cells at stage 9 in which apoptosis was induced.
E75A expression in the follicle cell under inducing Z2 and Z3 overexpression. (A) Results of in situ hybridization to the ovaries of hsZ2 and Z3 transgenic flies with adequate nutrition, with or without heat shock. (a, b) E75A expression in the adequate nutrition (F3) with heat shock (+hs). (c)E75A expression in the ovary of F3 female without heat shock (−hs). Number indicates the stage of oogenesis. (B) Results of RT-PCR, using total RNA, extracted from egg chambers (n=150 flies) at stages 8 and 9 only of fed (F3) and starved (F3S1) hsZ2 and hsZ3 transgenic flies with or without heat shock (39°C 30 min). (C) Percentage of stage 8 egg chambers in which E75A expression was induced in follicle cells and at stage 9 in which apoptosis was induced in the nurse cells of BR-C Z2 and Z3 transgenic flies (fed flies). The percentage was calculated as follows: {(number of egg chambers at stage 8, E75A expression in the follicle cell)/(total number of egg chambers at stage 8)−(number of stage 8 egg chambers showing nuclear condensation and fragmentation)} × 100. The percentage of the egg chambers (apoptosis in the nurse cells at stage 9) is calculated as follows: {(number of egg chambers at stage 9 indicate nuclear condensation and fragmentation in the nurse cells)/(total number of egg chambers at stage 9)} × 100. There were no significant differences between the percentage of egg chambers showing E75A expression in the follicle cells and the percentage of egg chambers showing apoptosis in nurse cells at stage 9 in each condition. *Indicates that there are significant differences (within 5%) comparing results with and without heat shock. n=12 flies
E75B expression patterns in the ovaries of hsZ2 and hsZ3 transgenic flies under fed conditions were the same as the ovaries of OrR flies under fed conditions, and when these transgenic flies underwent heat shock, the expression patterns did not differ from the flies without heat shock (data not shown). However, E75B expression levels were different with and without the heat shock inducing Z2 and Z3 overexpression (Figure 5) and decreased in the egg chambers at stages 8 and 9 under fed and starved conditions (Figure 5). Heat shock did not affect E75B expression at stages 8 and 9 in wild-type control flies.
E75B expression in the ovary following Z2 and Z3 overexpression. (a) Results of RT-PCR, demonstrating E75B expression, using total RNA from the stages 8 and 9 egg chambers of fed (F3) and starved (F3S1) hsZ2 and Z3 transgenic flies with or without heat shock (39°C 30 min). n=150 flies. (b) The graph indicates the expression levels with±S.D. (measured by NIH image). *Indicates that there is a significant difference (within 5%) comparing results with and without heat shock
Z2 and Z3 overexpression induced E75A expression and suppressed E75B expression in the egg chambers at stages 8 and 9. BR-C controls the developmental checkpoint in oogenesis at stages 8 and 9, by inducing apoptosis and regulating yolk protein gene expression.8 Probably, E75A and E75B expression in the follicle cells and nurse cells at stages 8 and 9 are regulated also by BR-C, in this case Z2 and Z3, and thus E75A and B expression in the follicle cells may ultimately induce the apoptosis of the nurse cells at stages 8 and 9, which leads to degeneration of the egg chamber. Apoptosis must therefore involve communication between the nurse cells, oocyte and follicle cells.
E75B suppresses E75A expression
We investigated whether or not E75A and E75B affected each other in the egg chambers at stages 8 and 9. E75A overexpression did not affect the E75B expression pattern in the ovary (data not shown), but E75B overexpression affected E75A (Figure 6A). In starved flies, E75A was expressed in the egg chambers at stages 8 and 9 of hsE75B transgenic flies under starvation in the same pattern as wild-type flies under starvation (Figure 6A b and c); but when the transgenic flies were heat shocked under starvation, E75A expression at stages 8 and 9 was not seen or was very faint (Figure 6A a). E75A overexpression did not affect E75B expression at stages 8 and 9 and E75B overexpression suppressed E75A expression in the egg chamber at stages 8 and 9 (Figure 6B, C and E). In starved wild-type flies, the percentage of egg chambers in which E75A expression was induced at stage 8 and in which apoptosis was induced at stage 9 was approximately 40%, but when E75B transgenic flies underwent heat shock to induce overexpression, this percentage was decreased to approximately 20% (Figure 6D). This is a significant drop in the number of egg chambers undergoing apoptosis.
E75A expression in Drosophila is inhibited by E75B overexpression. (A) Results of in situ hybridization to the RNA of ovaries of hsE75B transgenic flies (269) under nutritional shortage with or without heat shock. (a) E75A expression in the ovary under nutritional shortage (F3S1) with heat shock (+hs), resulting in E75B being expressed in nurse cells and follicle cells. (b and c) E75A expression in the ovary of S3 females without heat shock (−hs). The number on the panel indicates the stage of oogenesis. (B)Results of RT-PCR (E75A expression) using total RNA extracted from the egg chambers (n=150 flies) at stages 8 and 9 in E75B transgenic flies (fed, F3 and starved, F3S1 flies). (C) tResults of RT-PCR (E75B expression), using total RNA, extracted from the egg chambers (n=150 flies) at stages 8 and 9 in E75A transgenic flies (fed, F3 and starved, F3S1 flies). (D) Percentage of stage 8 egg chambers in which E75A expression was induced in the follicle cells and at stage 9 in which apoptosis was induced in the nurse cells of E75B transgenic flies (starved flies, line 267 and 269). There were no significant differences between the percentage of the egg chambers showing E75A expression in the follicle cells at stage 8 and the percentage of egg chambers showing E75A expression in the nurse cells at stage 9. *Means that there are significant differences (within 5%) comparing with and without heat shock. n=12 flies. (E) The graph indicates the E75B expression levels using RT-PCR, and total RNA from stages 8 and 9 egg chambers of E75B transgenic flies. The results are summarized in (C) showing relative value±S.D. (measured by NIH image). *Mean that there are significant differences between experiments with and without heat shock
Therefore, in the egg chamber at stages 8 and 9, E75B suppressed E75A expression in the follicle cells and nurse cells under fed and starved conditions. We propose that E75A expression in the follicle cells at stages 8 and 9 induces apoptosis in the nurse cells at these stages, but if E75B expression in the follicle cells at stages 8 and 9 is above threshold level, E75B suppresses E75A in the follicle cells, which in turn suppresses apoptosis in the nurse cells at stages 8 and 9.
Discussion
Early ecdysone response genes control apoptosis in nurse cells at stages 8 and 9
Apoptosis of many insect tissues and glands are affected by 20E and JH. 20E induces apoptosis and JH III treatment inhibits apoptosis in a Drosophila cell line, (l) 2 man.29 In Maduca sexta, 20E induces and JH suppresses the apoptosis of the prothoracic gland.30, 31 It is known that the early ecdysone response genes, BR-C, E74 and E75 are essential for the regulation of apoptosis in insect tissues and glands.18 E74A and BR-C activate the apoptosis inducer, hid, in the Drosophila salivary gland and BR-C activates reaper, also an apoptosis inducer.23 E75A and B induce apoptosis of salivary glands through suppression of diap2, an apoptosis suppressor.23
We have recently established that the BR-C controls the fate of the egg chamber, by progressing either development or apoptosis.8 The number of Drosophila egg chambers is reduced under nutritional shortage8 and in response to increasing ecdysone concentration.7 Apoptosis of the nurse cells at stages 8 and 9 causes a reduction in the number of egg chambers. Degenerating stages 8 and 9 egg chambers often appear elongated and the surface of the egg chamber has a rough appearance.7 In degenerating stages 8 and 9 egg chambers, follicle cells and their nuclei increase in size.7 Follicle cells are likely to phagocytose the dying nurse cells.3 After that the follicle cells die.7 Further, the apoptosis seems to be induced by BR-C Z2 and Z3 expression at stage 8. Nutritional shortage induced increased ecdysone concentration in flies;9 thus, it seemed likely that other ecdysone response genes may also affect apoptosis in the nurse cells at stages 8 and 9.
As shown in Table 1, E75A induces apoptosis in nurse cells at stages 8 and 9 of oogenesis. Stronger E75A expression in the follicle cells at stages 8 and 9 is observed in the egg chambers of females that are starved or injected with 20E, namely under apoptotic conditions (Figure 1A). In addition, BR-C, Z2 and Z3 (which induce apoptosis at stages 8 and 9) overexpression induced E75A expression at stages 8 and 9 (Figure 4). However, E75B does not act as an apoptosis inducer in the egg chamber. E75B overexpression suppresses apoptosis in the egg chambers of starved flies (Table 1). E75B expression levels at stages 8 and 9 reflect these results, E75B expression at stages 8 and 9 in fed and JHA- treated females (namely nonapoptotic conditions) is stronger than under apoptotic conditions (Figure 2B). In addition, E75B mutant flies were sensitive to nutritional shortage (Figure 3a). E75B mutant flies do not show abnormal development,14 but the percentage of egg chamber reduction in E75B mutants was higher during starvation than in wild-type females (Figure 3a). JHA treatment of starved flies suppresses the apoptosis of stage 8 and 9 egg chambers.7, 8 This suppression induced a recovery of the numbers of stage 8, 9 and 10 egg chambers (Figure 3b). JHA treatment induced higher E75B expression in stage 8 and 9 egg chambers (Figure 2B and C). On the other hand, starvation, 20E treatment and BR-C Z2 and Z3 overexpression in the follicle cells suppressed E75B expression in stage 8 and 9 egg chambers (Figures 2B, C and 5). Starvation induces high ecdysteroid concentrations in the haemolymph and ovary,32 which in turn may induce BR-C Z2 and Z3 expression in the follicle cells and thus induces apoptosis at stages 8 and 9.8 These results indicate that the two isoforms, E75A and E75B, have opposite effects on apoptosis of nurse cells. E75A acts as an apoptosis inducer and E75B acts as an apoptosis suppressor.
In summary, during apoptosis of the nurse cells at stages 8 and 9, BR-C Z2 and Z3 and E75A act as apoptosis inducers and E75B acts as an apoptosis suppressor as their expression is controlled in the follicle cells.
Interactions between ecdysone response genes
There is a developmental checkpoint at stages 8/9 in oogenesis.28 At this checkpoint, the egg chambers undergo a developmental selection, becoming committed to produce a mature egg or to undergo apoptosis. BR-C is a key gene at this developmental checkpoint. The progression of development or the induction of apoptosis depends on which BR-C isoforms are expressed in the follicle cells at stage 8.8 When flies are maintained under starvation conditions or 20E is injected into the abdomen, BR-C Z2 and Z3 are expressed in the follicle cells of the egg chamber at stage 8.8 In addition, BR-C Z1 and Z3 regulate yolk protein gene expression that begins at stage 8 in the ovary.8
BR-C Z2 and Z3 affect E75A and E75B expression at stages 8 and 9 (Figures 4 and 5). BR-C Z2 and Z3 overexpression induces stronger E75A expression in the egg chamber, both in nurse cells and follicle cells, at stages 8 and 9 under fed and starvation conditions (Figure 4A and B). In contrast, Z2 and Z3 overexpression suppresses E75B expression under fed and starved conditions (Figure 5). E75A and E75B are therefore downstream of the BR-C isoforms Z2 and Z3. Ecdysteroid concentration in starved flies is higher than in fed flies.9 We suggest that increasing ecdysteroid concentration induces BR-C Z2 and Z3 expression in the follicle cells at stages 8 and 9 and that Z2 and Z3 control the fate of egg chambers by regulating E75A and B expression in the follicle cells. Buszczak et al.14 show that BR-C, E75 and E74 are required for egg chamber development during mid-oogenesis. These genes are needed to permit the mature egg to develop, yet we find they are also needed to induce apoptosis. Expression of early ecdysone response genes is sensitive to changes in the ovarian ecdysone titre.14 Probably, there is a threshold of ecdysteroid titre in the ovary. Carney and Bender33 established that EcR is required for normal oogenesis in Drosophila. This means ecdysteroid is required for normal oogenesis in Drosophila. In contrast, ecdysteroids induce apoptosis at mid-oogenesis in Drosophila.8 We propose that these two opposite effects of ecdysteroid depend upon a threshold level of ecdysteroid titre. If levels are below the threshold, ecdysteroid induces the appropriate gene expression profile for normal oogenesis, but if it exceeds the threshold, ecdysteroids induce an alternative gene set that executes premature apoptosis at mid-oogenesis. Starvation would therefore cause the ecdysteroid concentration to exceed the threshold. We suggest that the ovarian ecdysone titre controls the apoptosis/development decision of individual egg chambers by regulation of the patterns of BR-C isoform expression.
A model for the network of ecdysone response genes, which control apoptosis in the nurse cells of the stages 8 and 9 egg chambers
Figure 7 proposes the first steps in a model for the ecdysone response gene network regulating apoptosis in the nurse cells of the egg chamber. In fed flies, only BR-C Z1 expression is observed in the follicle cells at stages 8 and 9;8 therefore, E75B expression in the follicle cells at stages 8 and 9 is not suppressed. E75B suppresses E75A expression in the follicle cells at stages 8 and 9, which in turn results in apoptosis of the nurse cells at these stages being suppressed and thus leads to the production of many mature eggs. When 20E is injected into the abdomen of fed flies, BR-C Z2 and Z3 are expressed in the follicle cells at stages 8 and 9. In fed flies, Z2 expression is induced by Z3 expression8 and it is possible that 20E activates Z2 expression directly. Z2 expression activates E75A expression in the follicle cells and suppresses E75B expression at stages 8 and 9. Z3 overexpression also activates E75A expression and suppresses E75B expression in the egg chamber at stages 8 and 9. However, we do not know if Z3 activates E75A and suppresses E75B by inducing Z2 expression or acts directly. E75A expression is increased in the follicle cells of the egg chamber at stages 8 and 9 by Z2, and E75A may activate the apoptosis pathway in the nurse cells and/or follicle cells at stages 8 and 9.
Model to explain the hierarchy of ecdysone response genes regulating apoptosis of stages 8 and 9 egg chambers. Complete nutrition induces normal development of the mature egg during oogenesis. In this case, just BR-C Z1 is expressed in the follicle cells at stage 8. E75B suppresses E75A expression in the follicle cells to prevent apoptosis. However, if 20E is injected into the abdomen of fed flies, Z2 and Z3 are expressed in the follicle cells at stage 8. In this case, Z2 expression in the follicle cells was induced by Z3 overexpression in the egg chambers. This results in a decrease in E75B and E75A induces apoptosis of the nurse cells at stages 8 and 9. Under starved conditions, Z2 and Z3 are also expressed in the follicle cells at stage 8 and suppress E75B and activate E75A expression in the follicle cells. As a result, the E75B expression level is not enough to suppress E75A expression, so E75A induces apoptosis in the nurse cells of the stages 8 and 9 egg chambers
In starved flies, nutritional shortage induces an increase in ecdysone concentration in flies9; ecdysone activates Z2 and Z3 expression in the egg chamber at stages 8 and 9.8 In this case, Z2 and Z3 expression do not affect each other; this suggests that ecdysone activates Z2 and Z3 expression in the follicle cells independently. Z2 and Z3 expression in the follicle cells activate E75A expression in the follicle cells at stages 8 and 9 and suppress E75B expression. As a result, E75A expression in the follicle cells should activate the apoptosis pathway in the nurse cells and/or follicle cells of the egg chamber and apoptosis commences at stages 8 and 9. Follicle cell development is partly independent of germ-line cell differentiation in Drosophila oogenesis,34 but induction of apoptosis at stages 8 and 9 under nutritional stress needs interactions between follicle cells and nurse cells.
The pattern of BR-C isoform expression in the follicle cells controls the checkpoint by interacting in each egg chamber to control a developmental switch leading to the development of a mature egg or to undergo apoptosis. BR-C expression is controlled by ecdysteroid concentration, which is increased in females under nutritional shortage.32 Ecdysteroids are crucial for normal oogenesis in Drosophila.14, 33, 35 On the other hand, our results suggest that ecdysteroids are also needed to induce apoptosis of the egg chamber at stages 8 and 9. We propose that there is a threshold ecdysteroid concentration in the fly and if ecdysteroid levels are below the threshold, Normal oogenesis is induced, but if levels exceed the threshold, ecdysteroids induce apoptosis of egg chambers at stages 8 and 9. When apoptosis of a nurse cell at stages 8 and 9 is induced, the genetic pathway that is activated differs between the fed and starved conditions. However, both pathways result in the activation of E75A expression in the follicle cells at stages 8 and 9 and the suppression of E75B expression at stages 8 and 9. E75A may activate the apoptosis pathway, for example the caspase pathway, which executes apoptosis. We do not know how the apoptosis that we observe in nurse cells is affected by what is happening to gene expression in the nurse cells. It is possible that E75A activates an apoptosis inducer in the follicle cells and the inducer activates the apoptosis pathway in the adjacent nurse cells or is transported to nurse cells so that both cell types die. We have identified some candidate genes for inducing or suppressing apoptosis by a microarray analysis.15 Our results show that E75A is an apoptosis inducer and E75B is the inhibitor. E75A may control expression of apoptosis inducers, which we identified by the microarray analysis, including Dp, p53 or the caspase family. On the other hand, E75B controls expression of an apoptosis inhibitor, and we therefore suggest this may be Diap1 and Diap2. The expression of E75A and E75B are regulated by alternative splicing in the yellow fever mosquito Aedes aegypti.36 We propose that the regulation of alternative splicing of E75 in the Drosophila ovary is controlled by BR-C Z2 and Z3 expression in the follicle cells of stages 8 and 9. The network of genes involved in controlling this developmental decision, which regulates how many eggs a female will produce, is complex and further investigations on how events are coordinated in the nurse cells, oocyte and follicle cells remain to be undertaken.
Materials and Methods
Drosophila maintenance
Flies were maintained on standard yeast, maize meal, sugar and agar medium at 25°C. The wild-type strain, Oregon R (OrR) was used throughout. All the flies for each experiment were 3 days old. Flies (3 days old) were transferred from a standard diet to one of sugar and water (starved, 1% agar medium, which contains 5% sucrose and 0.005% 10% Nipagen in 95% ethanol) or one of yeast (fed, 2 g bakers yeast on approximately 50 ml 1% agar medium, which contains 2.5% cornflour, 5% sucrose, 1.75% lypophilized yeast and 0.005% Nipagen in 95% ethanol). After 3 days on sugar or yeast, flies were dissected (sugar: S3, yeast: F3), transferred to sugar for 1 day after 3 days on yeast (F3S1), or topically treated with Methoprene and maintained on sugar and water for 1 day (F3JHS1), injected with 20E and maintained on yeast for 1 day (F3EF1). We used BR-C transgenic flies (TN-Q1-Q2-Z1, Z2, Z3 and Z4, kindly provided by C Bayer), E75A transgenic flies (line 283, 286), E75B transgenic flies and E75Δ1 and E75Δ51 to make E75B−/Df (line 267 and 269, E75A, E75B transgenic and E75Δ1 and E75Δ51 flies were kindly provided by CS Thummel). Flies carrying the E75Δ51 allele were crossed to E75Δ1 mutants. The flies were maintained at 25°C for 3 days with yeast (Fed, F3) or starved for 1 day after maintaining with yeast for 3 days (Starved, F3S1), then underwent heat shock at 39°C for 30 min and were maintained at 25°C for 6 h.
Injection of 20E and application of JHA
20E (Sigma) was dissolved in Insect Ringer's solution (130 mM NaCl, 4.7 mM KCl, 1.9 mM CaCl2) and 50 nl was injected at a concentration of 2 μg/ml.37 The concentration of 20E was determined according to Bownes.9 With a haemolymph volume of approximately 1 μl/female,37 100 pg 20E/female leads to a concentration of 2 × 10−7 M. Methoprene (ZR515, Zoecon) was applied topically to the ventral abdomen in 100 nl acetone. Methoprene diluted 1 : 100 in acetone corresponds to a concentration of about 1 μg/100 nl. Controls were also undertaken injecting Ringer's only and treating flies with acetone.
Hoechst staining
Ovaries were dissected in Insect Ringer's solution and fixed in 4% paraformaldehyde. After fixation and permeabilization in 1% Triton X-100 in PBS, ovaries were stained in 1 μM Hoechst in PBS for 5 min, then washed twice in PBS for 2 h and mounted in FISH medium (220 mM 1,4-diazabicyclo [2.2.2] octane, 90% glycerol, 100 mM Tris-HCl pH 8.5) and examined immediately using fluorescent filters.
RNA in situ hybridization
The protocol is based on the procedure previously described38 and modified as follows. The ovaries were dissected in Ringer's solution and fixed for 20 min in 4% p-formaldehyde in PBS. After rinsing the tissue in PBT, it was treated for 10 min in methanol/0.5 M EGTA, pH 8 (9 : 1). The ovaries can then be stored in methanol at −20°C for several months. The stored ovaries were rehydrated in PBT. The prehybridization was carried out for 1 h at 45°C in DNA hybrid (50% deionized formamide, 5 × SSC, 100 μg/ml sonicated salmon sperm DNA, 50 μg/ml Heparin, 0.1% Tween-20). The ovaries were hybridized overnight at 45°C in DNA hybrid containing digoxigenin-labeled probe (DIG-DNA labeling and detection kit, Boehringer Mannheim). For detection, a 1 : 1000 dilution of anti-DIG-AP-conjugated Ab was used. The staining reaction was performed in 100 mM Tris pH 9.5, 50 mM MgCl, 10 mM NaCl, 0.2% Tween-20, 8 mM levamisole, 4.5 μl/ml NBT and 3.5 μl/ml X-phosphate (Boeringer Mannheim) for 5 h. Anti-DIG-AP conjugate was preabsorbed with postfixed wild-type (Oregon R) ovaries at overnight 4°C. The ovaries were mounted in a mixture of PBS/glycerol (1 : 4) for microscopy. After the ovaries had been double stained using Hoechst and in situ hybridization to RNA, they were washed in PBS and stained in 1 μM Hoechst in PBS for 5 min, then washed twice in PBS for 2 h in the dark and mounted in FISH medium.
RNA extraction and RT-PCR
Transcript levels in ovaries were detected by reverse transcriptase (RT)-PCR as described previously.39 Total RNA was extracted from egg chambers at stages 8 and 9. The egg chambers at stages 8 and 9 were isolated from the ovary after dissection. The primer sequences are as below: E75A, forward 5′-TCAAGTGTCATTTCGAAGCCA-3′ and reverse 5′-AGATTGGCGATTTCCTTGTG-3′, E75B, forward 5′-GCTCTAGACACCAAAGCCATGTGCCGATCT-3′ and reverse 5′-GGCGCAGGAGATTGGCGATT-3′. The expression levels (relative value and standard deviation) were quantified by NIH image (downloaded from http://rsb.info.nih.gov/nih-image/download.html).
Abbreviations
- BR-C :
-
Broad-Complex
- JHA:
-
juvenile hormone analog
- 20E:
-
20-hydroxyecdysone
References
King RC (1970) The meiotic behavior of the Drosophila oocyte. Int. Rev. Cytol. 28: 125–168
Waddingston CH and Okada E (1960) Some degenerative phenomena in Drosophila ovaries. J. Embryol. Exp. Morphol. 8: 341–348
Giorgi F and Deri P (1976) Cell death in ovarian chambers of Drosophila melanogaster. J. Embryol. Exp. Morphol. 35: 521–533
Foley K and Cooley L (1998) Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development 125: 1075–1082
Gutzeit HO (1986) The role of microfilaments in cytoplasmic streaming in Drosophila follicles. J. Cell Sci. 80: 159–169
Cooley L, Nerheyen E and Ayers K (1992) Chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell 69: 173–184
Soller M, Bownes M and Kubli E (1999) Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 208: 337–351
Terashima J and Bownes M (2004) Translating available good into the number of eggs laid by Drosophila melanogaster. Genetics 167: 1711–1719
Bownes M (1989) The role of juvenile hormone, ecdysone and the ovary in the control of Drosophila vitellogenesis. J. Insect Physiol. 32: 493–501
Thomas HE, Stunnenberg HG and Stewart AF (1993) Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and Ultraspiracle. Nature 362: 471–475
Yao TP, Forman BM, Jiang Z, Cherbas L, Chen LD, MacKeown M, Cherbas P and Evans RM (1993) Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature 366: 476–479
DiBello PR, Withers DA, Bayer CA, Fristrom JW and Guild GM (1991) The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics 129: 385–397
Segraves WA and Hogness DS (1990) The E75 ecdysone-inducible gene responsible for the 75B early puff in Drosophila encodes two new members of the steroid receptor superfamily. Gene Dev. 4: 204–219
Buszczak M, Freemen MR, Carlson JR, Bender M, Cooley L and Segraves WA (1999) Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development 126: 4581–4589
Terashima J and Bownes M (2005) A microarray analysis of genes involved in relating egg production to nutritional intake in Drosophila melanogaster. Cell Death Differ. 12: 429–440
Deng WM and Bownes M (1997) Two signaling pathways specify localized expression of the Broad-Complex in Drosophila egg shell patterning and morphogenesis. Development 124: 4639–4647
Tzolovsky G, Deng WM, Schlitt T and Bownes M (1999) The function of the Broad-Complex during Drosophila oogenesis. Genetics 153: 1371–1383
Buszczak M and Segraves WA (2000) Insect metamorphosis: out with the old, in with the new. Curr. Biol. 10: R830–R833
Dorstyn L, Colussi PA, Quinn LM, Richardson H and Kumar S (1999) Dronc, an ecdysone-inducible Drosophila caspase. Proc. Natl. Acad. Sci. USA 96: 4307–4312
Cakouros D, Daish T, Martin D, Baehrecke EH and Kumar S (2002) Ecdysone-induced expression of caspase DRONC during hormone-dependent programmed cell death in Drosophila is regulated by Broad-Complex. J. Cell Biol. 157: 985–995
White K, Tahaoglu E and Steller H (1996) Cell killing by the Drosophila gene reaper. Science 271: 805–807
Abbott MK and Lengyel JA (1991) Embryonic head involution and rotation of male terminalia require the Drosophila locus head involution defective. Genetics 129: 783–789
Jiang C, Lamblin AFJ, Steller H and Thummel CS (2000) A steroid-triggered transcriptional hierarchy controls salivary gland cell death during Drosophila metmorphosis. Mol. Cell 5: 445–455
Duckett CS, Nava VE, Gedrich RW, Clem RJ, Van Dongen JL, Gilfillan MC, Shiels H, Hardwick JM and Thompson CB (1996) A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 15: 2685–2694
Woodard CT, Baehrecke EH and Thummel CS (1994) A molecular mechanism for the stage specificity of the Dorosphila prepupal genetic response to ecdysone. Cell 79: 607–615
White KP, Hurban P, Watanabe T and Hogness DS (1997) Coordination of Drosophila metamorphosis by two ecdysone-induced nuclear receptors. Science 276: 114–117
Bialecki M, Shilton A, Fichtenberg C, Segraves WA and Thummel CS (2002) Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev. Cell 2: 209–220
Wilson TG (1982) A correlation between juvenile hormone deficiency and vitellogenic oocyte degeneration in Drosophila melanogaster. Wilhelm Roux's Arch. 191: 257–263
Ress C, Hotmann M, Maas U, Sofsky J and Dorn A (2000) 20-hydroxyecdysone-induced differentiation and apoptosis in the Drosophila cell line, l(2)mbn. Tissue Cell 32: 464–477
Dai JA and Gilbert LI (1998) Juvenile hormone prevents the onset of programmed cell death in the prothoracic glands of Manduca sexta. Gen. Comp. Endocrinol. 109: 155–165
Dai JA and Gilbert LI (1999) An in vitro analysis of ecdysteroid-elicited cell death in the prothoracic gland of Maduca sexta. Cell Tissue Res. 297: 319–327
Terashima J, Takaki K, Sakurai S and Bownes M (2005) Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J. Endocrinol. In press
Carney GE and Bender M (2000) The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics 154: 1203–1211
Gutzeit HO and Strauß A (1989) Follicel cell development is partly independent of germ-line cell differentiation in Drosophila oogenesis. Roux. Arch. Dev. Boil. 198: 185–190
Riddiford LM (1993) Hormone receptor and the regulation of insect metamorphosis. Receptor 3: 203–209
Pierceall WE, Li C, Biran A, Miura K, Raikhel AS and Segraves WA (1999) E75 expression in mosquito ovary and fat body suggests reiterative use of ecdysone-regulated hierarchies in development and reproduction. Mol. Cell. Endocrinol. 150: 73–89
Soller M, Bownes M and Kubli E (1997) Mating and sex peptide stimulate the accumulation of yolk in oocyte of Drosophila melanogaster. Eur. J. Biochem. 243: 732–738
Tautz D and Pfeifle C (1989) A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98: 81–85
Hodgetts RB, Clark WC, O'Keefe SL, Schouls M, Guild GM and Kalm L (1995) Hormonal induction of Dopa decarboxylase in the epidermis of Drosophila is mediated by the Broad-Complex. Development 121: 3913–3922
Acknowledgements
This project was supported by the BBSRC and a University of Edinburgh Wellcome VIP award. We are grateful to Hilary Anderson for assistance with preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Edited by J Abrams
Rights and permissions
About this article
Cite this article
Terashima, J., Bownes, M. E75A and E75B have opposite effects on the apoptosis/development choice of the Drosophila egg chamber. Cell Death Differ 13, 454–464 (2006). https://doi.org/10.1038/sj.cdd.4401745
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401745
Keywords
This article is cited by
-
Microbes control Drosophila germline stem cell increase and egg maturation through hormonal pathways
Communications Biology (2023)
-
Evolution of sexually-transferred steroids and mating-induced phenotypes in Anopheles mosquitoes
Scientific Reports (2019)
-
Stress-induced reproductive arrest in Drosophila occurs through ETH deficiency-mediated suppression of oogenesis and ovulation
BMC Biology (2018)
-
Genetic tools to study juvenile hormone action in Drosophila
Scientific Reports (2017)
-
Steroid Hormones and the Physiological Regulation of Tissue-Resident Stem Cells: Lessons from the Drosophila Ovary
Current Stem Cell Reports (2017)