Abstract
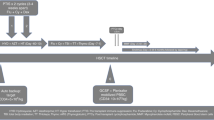
We developed a haploidentical transplantation protocol with post-transplant cyclophosphamide (CY) for in vivo T-cell depletion (TCD) using a novel adapted-dosing schedule (25 mg/kg on days +3 and +4) for Fanconi anemia (FA). With median follow-up of 3 years (range, 37 days to 6.2 years), all six patients engrafted. Two patients with multiple pre-transplant comorbidities died, one from sepsis and one from sepsis with associated chronic GVHD. Four patients without preexisting comorbidities and early transplant referrals are alive with 100% donor chimerism and excellent performance status. We conclude that adjusted-dosing post-transplant CY is effective in in vivo TCD to promote full donor engraftment in patients with FA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rosenberg PS, Tamary H, Alter BP . How high are carrier frequencies of rare recessive syndromes? Contemporary estimates for Fanconi Anemia in the United States and Israel. Am J Med Genet A 2011; 155a: 1877–1883.
Bonfim CM, de Medeiros CR, Bitencourt MA, Zanis-Neto J, Funke VA, Setubal DC et al. HLA-matched related donor hematopoietic cell transplantation in 43 patients with fanconi anemia conditioned with 60 mg/kg of cyclophosphamide. Biol Blood Marrow Transplant 2007; 13: 1455–1460.
Wagner JE, Eapen M, MacMillan ML, Harris RE, Pasquini R, Boulad F et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood 2007; 109: 2256–2262.
Zecca M, Strocchio L, Pagliara D, Comoli P, Bertaina A, Giorgiani G et al. HLA-haploidentical T cell-depleted allogeneic hematopoietic stem cell transplantation in children with Fanconi anemia. Biol Blood Marrow Transplant 2014; 20: 571–576.
Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 641–650.
Brodsky RA, Luznik L, Bolanos-Meade J, Leffell MS, Jones RJ, Fuchs EJ . Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant 2008; 42: 523–527.
Berger R, Bernheim A, Gluckman E, Gisselbrecht C . In vitro effect of cyclophosphamide metabolites on chromosomes of Fanconi anaemia patients. Br J Haematol 1980; 45: 565–568.
Thakar MS, Bonfim C, Sandmaier BM, O'Donnell P, Ribeiro L, Gooley T et al. Cyclophosphamide-based in vivo T-cell depletion for HLA-haploidentical transplantation in Fanconi anemia. Pediatr Hematol Oncol 2012; 29: 568–578.
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood 2003; 102: 2021–2030.
O'Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2002; 8: 377–386.
Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010; 115: 3224–3230.
Zanis-Neto J, Flowers MED, Medeiros CR, Bitencourt MA, Bonfim CM, Setúbal DC et al. Low-dose cyclophosphamide conditioning for haematopoietic cell transplantation from HLA-matched related donors in patients with Fanconi anemia. Br J Haematol 2005; 130: 99–106.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB et al. Cyclosporine plus methylprednisolone versus cyclophosphamide plus methylprednisolone as prophylaxis for graft-versus-host disease: a randomized double-blind study in patients undergoing allogeneic marrow transplantation. Clin Transplant 1987; 1: 21–28.
MacMillan ML, DeFor TE, Young JA, Dusenbery KE, Blazar BR, Slungaard A et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood 2015; 125: 3798–3804.
Acknowledgements
We thank the children with FA and their families for allowing us to care for them and for participating in our clinical trial. We are indebted to Dr Leo Luznik for our conversations regarding the modification of the posttransplant CY dosing. We give many thanks to our Clinical Research Coordinators at each site for data collection, management and integrity. We also thank Ms Helen Crawford and Ms Bonnie Larson for their assistance in manuscript preparation. Support for this study was provided by the Fanconi Anemia Research Fund (FARF) and by grants from the National Heart, Lung and Blood Institute (NHLBI), NIH, (Bethesda, MD, USA), numbers HL036444 and HL122173. HPK is a Markey Molecular Medicine Investigator and received support as the inaugural recipient of the José Carreras/E.D. Thomas Endowed Chair for Cancer Research.
Author contributions
MT designed the trial, wrote the protocol, enrolled patients, analyzed the data and wrote and edited the manuscript. CB, MW and RP enrolled patients, analyzed data and edited the manuscript. RS designed the trial and edited the manuscript. BS designed the trial, analyzed the data, enrolled patients and edited the manuscript. LB analyzed the data and edited the manuscript. AW and H-PK designed the trial, analyzed the data and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Thakar, M., Bonfim, C., Walters, M. et al. Dose-adapted post-transplant cyclophosphamide for HLA-haploidentical transplantation in Fanconi anemia. Bone Marrow Transplant 52, 570–573 (2017). https://doi.org/10.1038/bmt.2016.301
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.301