Abstract
The most common means of mobilizing autologous stem cells is G-CSF alone or combined with cyclophosphamide (CY) to obtain sufficient CD34+ cells for one to two transplants. There are few prospective, randomized studies investigating mobilization regimens in multiple myeloma (MM), especially after lenalidomide-based induction. We designed this prospective, randomized study to compare low-dose CY 2 g/m2+G-CSF (arm A) and G-CSF alone (arm B) after lenalidomide-based up-front induction in MM. Of the 80 initially randomized patients, 69 patients were evaluable, 34 and 35 patients in arms A and B, respectively. The primary end point was the proportion of patients achieving a yield of ⩾3 × 106/kg CD34+ cells with 1−2 aphereses, which was achieved in 94% and 77% in arms A and B, respectively (P=0.084). The median number of aphereses needed to reach the yield of ⩾3 × 106/kg was lower in arm A than in arm B (1 vs 2, P=0.035). Two patients needed plerixafor in arm A and five patients in arm B (P=0.428). Although CY-based mobilization was more effective, G-CSF alone was successful in a great majority of patients to reach the defined collection target after three cycles of lenalidomide-based induction.
Similar content being viewed by others
Introduction
Autologous stem cell transplantation (ASCT) as first line therapy is still the backbone in the treatment of younger patients with multiple myeloma (MM).1, 2 There are only few prospective, randomized trials comparing different mobilization methods in MM.3, 4 Early trials showed some benefit from high (7 g/m2) or intermediate CY (3−4 g/m2) compared with low-dose CY (1.5−2 g/m2) in terms of total CD34+ cell yields but with increased toxicity.5, 6, 7, 8 It has been assumed that CY would also benefit the outcome in patients with inferior response before mobilization but subsequent studies have not confirmed this.7, 9 With novel agents, it is possible to achieve at least a very good partial response for 55−75% of patients before mobilization.1
Prolonged exposure to lenalidomide may impair the mobilization of CD34+ cells without impact on engraftment kinetics.10, 11, 12, 13 If stem cell mobilization is scheduled in the early phase (⩽3−4 cycles of lenalidomide-based induction), the rate of failure to achieve grafts for 1−2 transplants is diminished.14, 15 The possible negative effect of lenalidomide on successful harvesting could be overcome by adding CY or plerixafor to G-CSF.16, 17, 18 The International Myeloma Working Group suggested that G-CSF alone would be adequate for initial mobilization in MM patients aged <65 years with fewer than four cycles of lenalidomide but encourages prospective trials investigating the up-front use of plerixafor.19 The American Society for Blood and Marrow Transplantation has published guidelines for autologous stem cell mobilization20, 21 and recommended early collection between the second and fourth cycles of lenalidomide whenever possible.20 The phase III study published by DiPersio et al.22 showed that G-CSF+plerixafor was superior to G-CSF+placebo in MM in terms of optimal CD34+ cell yield and the number of apheresis needed. Of note, only <10% of patients had received lenalidomide before mobilization in that trial. In a recent paper of Clark et al.,23 the prior therapies correlated with the risk of mobilization failure in the group mobilized with chemotherapy plus G-CSF but not in the plerixafor+G-CSF group. Mohty et al.24 concluded that preemptive intervention based on the CD34+ cell count prior to apheresis might help to rescue the mobilization failure.
The present phase II randomized prospective multicenter mobilization study was designed as a substudy in the Finnish Myeloma Study Group-MM02 trial to compare the efficacy of low-dose CY 2 g/m2+G-CSF vs G-CSF alone after lenalidomide, bortezomib and dexamethasone (RVD) induction. To the best of our knowledge, this is the first prospective randomized trial on stem cell mobilization after RVD induction.
Materials and methods
Study design
This phase II multicenter trial was conducted at 12 centers in Finland. Transplant-eligible patients aged ⩽70 years with untreated symptomatic MM were randomized 1:1 at inclusion into one of the two mobilization arms. Computer-generated random arms were assigned to patients. Principal investigator was responsible for inclusion and randomization. The main exclusion criteria were peripheral neuropathy grade ⩾2, significant liver dysfunction, severe cardiac dysfunction, severe renal failure (glomerular filtration rate <15 ml/min, unless in hemodialysis) and contraindication for the use of thromboprophylaxis or history of active malignancy during the past 5 years, with the exception of basal cell carcinoma of the skin or stage 0 cervical carcinoma. The Research Ethics Committee of the Northern Savo Hospital District approved the study protocol, and it was conducted according to the Declaration of Helsinki, International Conference of Harmonization and Guidelines for Good Clinical Practice. Written informed consent was obtained from all patients before inclusion.
The patients were initially treated with RVD induction comprising three 21-day cycles of lenalidomide 25 mg on days 1−14, bortezomib 1.3 mg/m2 on days 1, 4, 8, 11 subcutaneously and dexamethasone 20 mg/day on days 1−2, 4−5, 8−9 and 11−12. The mobilization in arm A was CY 2 g/m2 on day +1 plus filgrastim 5 μg/kg starting on day +4 and in arm B filgrastim 10 μg/kg alone starting on day +1. The apheresis was scheduled to begin if the blood CD34+ cell level was >10 × 106/L on day +10 or on day +5 in arms A and B, respectively. The second apheresis was started if the yield of the first apheresis was not ⩾3 × 106/kg for one or ⩾6 × 106/kg for double graft. Plerixafor was started at 10−11 PM hours with a dose of 240 μg/kg, if blood CD34+ level was <10 × 106/L in both arms provided that WBC count was ⩾10 × 109/L in arm A or day +5 had been achieved in arm B. Plerixafor was also scheduled if the yield of the first apheresis was <1 × 106/kg CD34+ cells. If plerixafor was started on these criteria, it was continued until the predetermined CD34+ cell number (⩾ 3 × 106/kg for a single transplant, ⩾6 × 106/kg for patients with an option for two transplants) was achieved.
The stem cell aphereses were centralized in the collection units at five university hospitals in Finland. Of all procedures, 90% were performed using the Spectra Optia MNC Program (Spectra Optia Apheresis System, Software 7.2, Terumo BCT Lakewood, CO, USA) processing 2.3−3 times total blood volume. The remaining 10% of the collections were performed using Fresenius COM.TEC (Blood Cell Separator Fresenius Hemo Care GmbH, Bad Homburg, Germany). All five flow cytometry laboratories participate in CD34+ Stem Cell Enumeration trials organized by UK NEQAS (www.ukneqasli.co.uk) six times a year with concordant results. The number of grafts to be collected for each patient was determined in advance in each study center. After collection, the patients received a single ASCT after melphalan 200 mg/m2. The use of G-CSF was recommended after the graft infusion on day +5 onwards if the number of CD34+ cells collected was <3 × 106/kg.
Study end points
The primary end point of the mobilization study was the proportion of patients with a yield ⩾3 × 106/kg for one transplant with 1−2 aphereses. Secondary end points were the number of aphereses needed to reach a yield ⩾3 × 106/kg (⩾6 × 106/kg for double graft), the need for plerixafor use and the proportion of patients reaching ⩾2 × 106/kg CD34+ cells (minimum collection target) with ⩽3 aphereses. This study was approved by the Finnish Medicines Agency and registered at www.clinicaltrials.gov as #NCT01790737.
Statistics
This was a phase II study where the immunophenotypic remission rate during protocol treatment was considered as the primary end point for sample size calculation (n=80) for the entire study. With regard to this mobilization substudy, we hypothesized that 90% of patients in arm A would achieve the defined goal without plerixafor compared with 60% of patients in arm B. With this hypothesis, the sample size of 32 patients per arm would be needed to show statistically significant difference between arms CY+G-CSF compared with G-CSF alone mobilization with 80% power and with 5% alpha error. Analyses were performed in all patients and between the two mobilization groups. All calculations and statistical analyses were conducted using the appropriate software (IBM SPSS Statistics 22.Ink, Chicago, IL, USA). Comparisons between the two study arms regarding continuous variables were performed using Mann–Whitney U-test and variation inside the groups was described in ranges. Fisher's exact test was used to compare the categorical variables between the study arms. Continuous variables were presented in medians with ranges and categorical variables in percentages. All P-values were two-tailed and values <0.05 were considered statistically significant.
Results
Mobilization of CD34+ cells
From January 2013 to February 2015, altogether 80 MM patients were included and randomized. By 18th of May 2015, 69 patients, 34 in arm A and 35 in arm B, were mobilized and harvested. Eleven patients (14%) were dropped out early before mobilization and collection due to toxicity (n=9) or early progression (n=2) (Supplementary Information, Consortium Flowchart). The patient characteristics are presented in Table 1. Blood CD34+ cell counts on the first apheresis day were similar between the arms, with medians of 43 (12−258) × 106/L and 39 (12−149) × 106/L, respectively (P=0.719; Table 2). A statistical difference emerged in the median number of blood CD34+ cells before the second collection, 45 (9−140) × 106/L and 33 (18−95) × 106/L, (P=0.032; Figure 1). The median of the blood CD34+ cell peak was 67 (14−258) × 106/L and 44 (18–149) × 106/L (P=0.106) and this was achieved on day +11 (10–15) and +5 (4–7) in arms A and B, respectively.
Collection of CD34+ cells
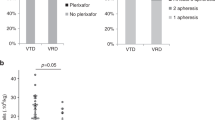
There was no statistical difference between arms A and B with regard to the primary study end point, that is, the proportion of patients achieving a yield of at least ⩾3 × 106/kg with 1−2 aphereses (94 and 77%, respectively, P=0.084; Table 2). Arm A was superior regarding one of the secondary end points namely the number of aphereses needed to reach yield ⩾3 × 106/kg, 1 vs 2, P=0.035. The double graft goal (⩾6 × 106/kg) was predetermined for 21/34 (62%) and 18/35 (51%) of patients in arms A and B, respectively (P=0.469). The proportion of patients able to achieve this goal with 1−2 aphereses was 62% in arm A and 50% in arm B (P=0.662). All patients in both arms reached the secondary end point, a yield of ⩾2 × 106/kg CD34+ cells (minimum collection target) with ⩽3 aphereses. The total number of CD34+ cells collected was higher after CY 2 g/m2+G-CSF than after G-CSF alone, with medians of 6.7 (2.2−12.4) × 106/kg and 5.3 (2.4−12.4) × 106/kg, respectively (P=0.012). Plerixafor was needed for 2 (6%) patients in arm A and for 5 (14%) patients in arm B (P=0.428).
There was a statistically significant difference between the arms regarding the yield of the first apheresis after CY+G-CSF (median 4.0 (0.8−12.4) × 106/kg) and after G-CSF (2.7 (0.5−12.4) × 106/kg) (P=0.023; Table 2). The median CD34+ cell yields in both arms per apheresis are shown in Figure 2. There was no difference between total blood volume processed between study arms (P=0.841). There was no statistically significant difference with respect to the yield ⩾4 × 106/kg (target for a single transplant suggested by International Myeloma Working Group19) with one apheresis; 17/34 (50%) achieved it in arm A compared with 10/35 (29%) in arm B, P=0.057. Days of hospitalization as well as toxicity during mobilization and apheresis are shown in Table 3.
Transplantation and engraftment
There was a statistical difference between the arms regarding the number of CD34+ cells infused after high-dose melphalan 4.3 (2.2−7.3) × 106/kg and 3.2 (2.3−6.2) × 106/kg in arms A and B, respectively (P=0.010). The engraftment kinetics were, however, similar regarding the recovery of neutrophil counts >0.5 × 109/L (days +14 (9−28) and +14 (11−27), P=0.879) and platelet counts >20 × 109/L without platelet infusions (days +12 (8−30) and 11 (8−30), P=0.672) and blood counts on day +15 (except the lymphocyte count difference, 0.5 × 109/L (0.1−2.8) and 0.7 × 109/L (0.2−2.6), P=0.019) in arms A and B, respectively. Use of G-CSF after graft infusion was equal in both arms (43% and 40% in arms A and B, respectively, P=1.000). The median recovery of neutrophils appeared on day +12 (11−19) in patients with CD34+ cells infused <3 × 109/L who had G-CSF support by the protocol and on day +14 (9−28) in patients with CD34+ cell count ⩾3 × 109/L without G-CSF support after ASCT. There was no difference between the arms in hospitalization days during ASCT (Table 3). No early deaths owing to infections or any other causes were observed in transplanted patients with at least short-term follow-up. There were fewer patients with neutropenic fever during ASCT in the G-CSF arm. There was no difference in the need for supportive care during ASCT according to the mobilization arms.
Discussion
ASCT remains the standard up-front treatment for MM patients at least until the results of two large randomized prospective multicenter trials comparing ASCT with novel agents and early vs delayed ASCT have been published.25, 26 On the other hand, debate continues regarding a double graft option for MM patients aged <65−70 years. After VAD induction, very few patients failed to mobilize an adequate number of CD34+ cells for double transplantation.27 In the era of novel agents, some concern has been raised regarding the adequacy of the stem cell yields after lenalidomide-based induction. Our randomized study showed that although CY-based mobilization was more effective G-CSF alone was successful in a great majority of patients to reach the defined collection target after a short course of lenalidomide-based induction.
The mechanisms behind the mobilization problems after lenalidomide have been investigated by the groups of Koh et al.28 and Pal et al.,29 who found a maturation arrest of neutrophils causing the upregulation of intrinsic G-CSF. Based on that, patients could develop tachyphylaxis for the use of G-CSF and finally end in failure in the harvest phase. Lenalidomide seems to induce localization of C-X-C chemokine receptor 4 (CXCR4) in the cell surface and increase the binding of CXCR4 to stromal-derived factor-1α, which blocks the mobilization of CD34+ cells and could be overcome using plerixafor.30 In contrast to lenalidomide, bortezomib has been demonstrated to have some enhancing effects with CY+filgrastim mobilization,31, 32 and a pilot study on filgrastim plus bortezomib mobilization has been registered.33 In our previous study34 of bortezomib-based (VD) induction followed by low-dose CY 2 g/m2+G-CSF mobilization, the yields were about 30% higher (9.9 × 106/kg (2.9−14.6) with a median of two aphereses) than in this study, suggesting some detrimental effects even of a short course of lenalidomide in terms of CD34+ cell mobilization.
In the present study, the median CD34+ cell yields after CY+G-CSF mobilization were comparable with those in other studies using RVD as induction.14, 15 There are no data for comparison with regard to RVD induction followed by mobilization with G-CSF alone. The highest blood CD34+ cell counts in our CY+G-CSF arm were slightly lower than those seen after VAD induction followed by CY+G-CSF mobilization, even if the response to induction is better with the novel induction treatments.6 The lower blood CD34+ cell counts after lenalidomide exposure can usually be successfully compensated by the use of plerixafor.18
In our randomized mobilization study, we observed that the percentage of patients reaching the primary end point was similar after CY+G-CSF compared with G-CSF alone mobilization regimen, and all patients achieved the minimum collection target at first attempt. Because in our study the accepted goal for one graft was ⩾3 × 106/kg instead of the usual recommendation of ⩾4 × 106/kg,19, 21 we analyzed whether there would have been a difference in toxicity during ASCT between the patient groups having received CD34+ cells <4 or ⩾4 × 106/kg after MEL200. There was no statistically significant difference in terms of neutropenic fever, number of red cell or platelet transfusions, neutrophil or platelet engraftment or hospitalization during ASCT. In the CY arm, CD34+ cell yields were higher after the first and the second harvests even if blood CD34+ levels were higher only before second harvest. On the other hand, patients in CY+G-CSF arm had three extra hospital days compared with G-CSF alone arm based on long distances to the hospitals in Finland.
In conclusion, low-dose CY+G-CSF is more effective than G-CSF alone in autologous stem cell mobilization in MM patients in terms of the number of aphereses needed and graft CD34+ content. However, G-CSF alone mobilization could be an alternative after induction with three cycles of RVD to harvest even for a double transplant program. Plerixafor was needed for 6% of the patients after CY+G-CSF and for 14% of patients in the G-CSF arm. In all of these patients, the graft could be successfully collected without need for a second mobilization attempt. Based on these results with limited number of patients, CY 2 g/m2 might be omitted in mobilization in MM patients, at least after three-cycle RVD induction. However, if the goal were ⩾4 × 106/kg19, 21 for one graft and the number of grafts to be collected were two for a younger myeloma patient the best regimen at this moment may still be CY+G-CSF+/−plerixafor up-front.
Our next step will be a comparison of the graft cellular compositions and immune reconstitution after high-dose therapy between these mobilization arms as well as costs associated with the mobilization and collection phases between the arms. These aspects will be of importance with regard to optimizing mobilization strategies in myeloma patients scheduled for ASCT.
References
Stewart AK, Richardson PG, San Miguel JF . How I treat multiple myeloma in younger patients. Blood 2009; 114: 5436–5443.
Gertz MA, Dingli D . How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood 2014; 124: 882–890.
Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol 1998; 16: 1547–1553.
Sheppard D, Bredeson C, Allan D, Tay J . Systematic review of randomized controlled trials of hematopoietic stem cell mobilization strategies for autologous transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2012; 18: 1191–1203.
Fitoussi O, Perreau V, Boiron JM, Bouzigon E, Cony-Makhoul P, Pigneux A et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant 2001; 27: 837–842.
Jantunen E, Putkonen M, Nousiainen T, Pelliniemi T-T, Mahlamäki E, Remes K . Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilization in patients with multiple myeloma. Bone Marrow Transplant 2003; 31: 347–351.
Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzov MR et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma 2006; 6: 384–388.
Hiwase DK, Bollard G, Hiwase S, Bailey M, Muirhead J, Schwarer AP . Intermediate-dose CY and G-CSF more efficiently mobilize adequate numbers of PBSC for tandem autologous PBSC transplantation compared with low-dose CY in patients with multiple myeloma. Cytotherapy 2007; 9: 539–547.
Bacon WA, Long GD, Rizzieri DA, Horwitz ME, Chute JP, Sullivan KM et al. Impact of high-dose cyclophosphamide on the outcome of autologous stem cell transplant in patients with newly diagnosed multiple myeloma [abstract]. (ASH Annual Meeting Abstracts). Blood 2011; 118: 4127.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007; 21: 2035–2042.
Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S . Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia 2008; 22: 1280–1281.
Paripati H, Stewart AK, Cabou S, Dueck A, Zepeda VJ, Pirooz N et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia 2008; 22: 1282–1284.
Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant 2009; 15: 718–723.
Richardson PG, Weller W, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS et al. Lenalidomide, bortezomib and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010; 116: 679–686.
Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by Intergroupe Francophone du Myelome. J Clin Oncol 2014; 32: 2712–2718.
Mark T, Stern J, Furst JR, Jayabalan D, Zafar F, LaRow A et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant 2008; 14: 795–798.
Cavallo F, Bringhen S, Milone G, Ben-Yehuda D, Nagler A, Calabrese E et al. Stem cell mobilization in patients with newly diagnosed multiple myeloma after lenalidomide induction therapy. Leukemia 2011; 25: 1627–1631.
Costa LJ, Abbas J, Hogan KR, Kramer C, McDonald K, Butcher CD et al. Growth factor plus preemptive ('just-in-time') plerixafor successfully mobilizes hematopoietic stem cells in multiple myeloma patients despite prior lenalidomide exposure. Bone Marrow Transplant 2012; 47: 1403–1408.
Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood 2009; 114: 1729–1735.
Giralt S, Costa L, Schriber J, DiPersio J, Maziarz R, McCarty J et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant 2014; 20: 295–308.
Duong HK, Savani BN, Copelan E, Devine S, Costa LJ, Wingard JR et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2014; 20: 1262–1273.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef INM, Stiff PJ, Kaufman JL et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009; 113: 5720–5726.
Clark RE, Bell J, Clark JO, Braithwaite B, Vithanarachchi U, McGinnity N et al. Plerixafor is superior to conventional chemotherapy for first-line stem cell mobilization, and is effective even in heavily pretreated patients. Blood Cancer J 2014; 4: e255.
Mohty M, Hübel K, Kröger N, Aljurf A, Apperley J, Basak GW et al. Autologous haematopoietic stem cell mobilization in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2014; 49: 865–872.
Stichting Hemato-Oncologie voor Volwassenen Nederland. Study to compare VMP with HDM followed by VRD consolidation and lenalidomide maintenance in patients with newly diagnosed multiple myeloma (HO95). Available at http://clinicaltrials.gov NCT01208766.
Richardson PG . Dana-Farber Cancer Institute. Randomized trial of lenalidomide, bortezomib, dexamethasone vs. high-dose treatment with SCT in MM patients up to age 65 (DFCI 10-106). Available at http://clinicaltrials.gov. NCT01208662.
Moreau P, Hulin C, Marit G, Gaillon D, Facon T, Lenain P et al. Stem cell collection in patients with de novo multiple myeloma treated with the combination of bortezomib and dexamethasone before autologous stem cell transplantation according to IFM 2005-01 trial. Leukemia 2010; 24: 1233–1235.
Koh KR, Janz M, Mapara MY, Lemke B, Stirling D, Dörken B et al. Immunomodulatory derivative of thalidomide (IMiD CC-4407) induces a shift in lineage commitment by suppressing erythropoiesis and promoting myelopoiesis. Blood 2005; 105: 3833–3840.
Pal R, Monaghan SA, Hassett AC, Mapara MY, Schafer P, Roodman GD et al. Immunomodulatory derivatives induce PU.1 down-regulation, myeloid maturation arrest, and neutropenia. Blood 2010; 115: 605–614.
Li S, Fu J, Ma H, Mapara MY, Lentzsch S . Lenalidomide-induced upregulation of CXCR4 in CD34+ hematopoietic cells, a potential mechanism of decreased hematopoietic progenitor mobilization. Leukemia 2013; 27: 1407–1411.
Hopman RK, DiPersio JF . Advances in stem cell mobilization. Blood Rev 2014; 28: 31–40.
Niesvizky R, Mark TM, Ward M, Jayabalan DS, Pearse RN, Manco M et al. Overcoming the response plateau in multiple myeloma: a novel bortezomib-based strategy for secondary induction and high-yield CD34+ stem cell mobilization. Clin Cancer Res 2013; 19: 1534–1546.
A pilot study of peripheral blood hematopoietic stem cell mobilization with the combination of bortezomib and G-CSF in multiple myeloma and non-Hodgkin’s lymphoma patients. Available at http://clinicaltrials.gov NCT02037256.
Silvennoinen R, Kairisto V, Pelliniemi T-T, Putkonen M, Anttila P, Säily M et al. Assessment of molecular remission rate after bortezomib plus dexamethasone induction treatment and autologous stem cell transplantation in newly diagnosed multiple myeloma patients. Br J Haematol 2013; 160: 561–564.
Acknowledgements
We thank the Research Committee of the Kuopio University Hospital Catchment Area for State Research Funding (project 5101424, Kuopio, Finland). This study was supported by research funding from Celgene. Celgene provided the study drug lenalidomide. We thank all the patients for participating in this study.
Author contributions
RS, EJ, PA, MS, AR and TL designed this study. PA, JH, MS, TMS, SK, HO, MP, VT, AK, KL, AR, AS, MS, PB, KK, TK, KR, EJ and RS provided research material or patients for the study. RS and TS performed the statistical analyses. RS wrote the manuscript, EJ and KR revised the manuscript. All authors edited and approved the manuscript. The authors take full responsibility for the content of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
RS has received a research grant from Celgene and Janssen and honoraria from Genzyme and Sanofi. EJ has received honoraria from Genzyme and Sanofi and has participated in an EU Leadership meeting organized by Genzyme as well as a Medical Advisory Board meeting organized by Genzyme. KR has received Advisory Board honoraria from Celgene, Janssen and Roche. MP has received Advisory Board honoraria from Amgen and Celgene. KK has received honoraria from Amgen, Roche and Fresenius and educational travel grants from Amgen and Hospira. AR has participated in Advisory Boards organized by Amgen and Roche. MS has received educational travel grants from Celgene, Genzyme and Pfizer. KL has received educational travel grants from Celgene, Mundipharma and Novartis. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Silvennoinen, R., Anttila, P., Säily, M. et al. A randomized phase II study of stem cell mobilization with cyclophosphamide+G-CSF or G-CSF alone after lenalidomide-based induction in multiple myeloma. Bone Marrow Transplant 51, 372–376 (2016). https://doi.org/10.1038/bmt.2015.236
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.236
This article is cited by
-
Efficacy of hematopoietic stem cell mobilization regimens in patients with hematological malignancies: a systematic review and network meta-analysis of randomized controlled trials
Stem Cell Research & Therapy (2022)
-
Comparison of the efficiency, safety, and survival outcomes in two stem cell mobilization regimens with cyclophosphamide plus G-CSF or G-CSF alone in multiple myeloma: a meta-analysis
Annals of Hematology (2021)
-
Initial Therapeutic Approaches to Patients with Multiple Myeloma
Advances in Therapy (2021)
-
The association of mobilising regimen on immune reconstitution and survival in myeloma patients treated with bortezomib, cyclophosphamide and dexamethasone induction followed by a melphalan autograft
Bone Marrow Transplantation (2021)
-
Bortezomib and cyclophosphamide based chemo-mobilization in multiple myeloma
International Journal of Hematology (2020)