Abstract
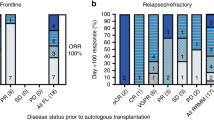
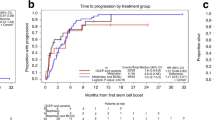
Cyclophosphamide, bortezomib and dexamethasone (CyBorD) is a highly active three-drug induction regimen for untreated transplant-eligible multiple myeloma patients. Although CyBorD has been evaluated only in the phase 2 setting in a limited number of patients, its high efficacy and ease of administration have led to its widespread use. Given that clinical trial efficacy can overestimate real-life effectiveness, we reviewed our institutional experience with 109 newly diagnosed patients who were treated with CyBorD in a non-clinical trial setting. After a median of four cycles, overall response rate (ORR) and very good partial response rate or better (⩾VGPR) were 95 and 66%, respectively, comparable to phase 2 studies of CyBorD and other three/four-drug induction regimens. All patients subsequently underwent successful stem cell collection and upgraded responses to ORR 98% and ⩾VGPR 79% post transplant. At a median follow-up of 19.8 months after diagnosis, the 2-year OS probability was 95.3% (95%CI: 89–98). The presence of concurrent plasmacytoma at diagnosis was the only prognostic factor predicting poorer survival (HR=5.56; 95%CI: 0.92–33.74; P=0.03). CyBorD was well-tolerated, with no severe peripheral neuropathy and minimal hematologic toxicity. Therefore, CyBorD is a convenient, well-tolerated, highly effective induction regimen in preparation for autologous SCT in real-life clinical practice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol 2010; 28: 4621–4629.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010; 11: 29–37.
Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 2010; 376: 2075–2085.
Gay F, Hayman SR, Lacy MQ, Buadi F, Gertz MA, Kumar S et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood 2010; 115: 1343–1350.
Kaufman JL, Nooka A, Vrana M, Gleason C, Heffner LT, Lonial S . Bortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myeloma: a retrospective study. Cancer 2010; 116: 3143–3151.
Lacy MQ, Gertz MA, Dispenzieri A, Hayman SR, Geyer S, Kabat B et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc 2007; 82: 1179–1184.
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 2009; 23: 1337–1341.
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Laumann K et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood 2010; 115: 3416–3417.
Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010; 116: 679–686.
Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood 2012; 120: 1589–1596.
Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol 2012; 30: 2946–2955.
Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012; 119: 4375–4382.
Kumar SK, Lacy MQ, Hayman SR, Stewart K, Buadi FK, Allred J et al. Lenalidomide, cyclophosphamide and dexamethasone (CRd) for newly diagnosed multiple myeloma: results from a phase 2 trial. Am J Hematol 2011; 86: 640–645.
Reeder CB, Reece DE, Kukreti V, Mikhael JR, Chen C, Trudel S et al. Long-term survival with cyclophosphamide, bortezomib and dexamethasone induction therapy in patients with newly diagnosed multiple myeloma. Br J Haematol 2014; 167: 563–565.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Ludwig H, Viterbo L, Greil R, Masszi T, Spicka I, Shpilberg O et al. Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. J Clin Oncol 2013; 31: 247–255.
Mateos MV, San Miguel JF . Safety and efficacy of subcutaneous formulation of bortezomib versus the conventional intravenous formulation in multiple myeloma. Ther Adv Hematol 2012; 3: 117–124.
Ng P, Incekol D, Lee R, Paisley E, Dara C, Brandle I et al. Tolerability of Velcade (Bortezomib) subcutaneous administration using a maximum volume of 3 mL per injection site. J Oncol Pharm Pract 2014; 29: 29.
Chanan-Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA . Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res 2012; 18: 2145–2163.
Dimopoulos MA, Roussou M, Gkotzamanidou M, Nikitas N, Psimenou E, Mparmparoussi D et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia 2013; 27: 423–429.
Kumar SK, Engebretson AE, Buadi FK, Lacy MQ, Dispenzieri A, Duh MS et al. Comparable Outcomes With Bortezomib-Cyclophosphamide-Dexamethasone (VCD) and Bortezomib-Lenalidomide-Dexamethasone (VRD) For Initial Treatment Of Newly Diagnosed Multiple Myeloma (MM). Blood 2013; 122: 3178.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CC received honoraria from Janssen and Celgene. ST received honoraria from Celgene. VK received honoraria form Celgene and Lundbeck. RT received honoraria from Janssen and Celgene. DR received research funds from Millinneum, Janssen and Celgene, honoraria from Janssen, Celgene and Amgen, Consulting fees from Janssen and Celgene. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Areethamsirikul, N., Masih-Khan, E., Chu, CM. et al. CyBorD induction therapy in clinical practice. Bone Marrow Transplant 50, 375–379 (2015). https://doi.org/10.1038/bmt.2014.288
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.288
This article is cited by
-
Weekly cyclophosphamide, subcutaneous bortezomib and dexamethathasone (CyBorD) for initial treatment of transplant-eligible patients with multiple myeloma: experience of two transplant centres
Bone Marrow Transplantation (2021)
-
Toxicity and survival outcomes of autologous stem cell transplant in multiple myeloma patients with renal insufficiency: an institutional comparison between two eras
Bone Marrow Transplantation (2020)
-
The effect of pamidronate delivery in bisphosphonate-naïve patients on neutrophil chemotaxis and oxidative burst
Scientific Reports (2020)
-
Bortezomib-containing regimens (BCR) for the treatment of non-transplant eligible multiple myeloma
Annals of Hematology (2017)
-
Bortezomib-thalidomide-dexamethasone (VTD) is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) as induction therapy prior to autologous stem cell transplantation in multiple myeloma
Leukemia (2015)