Abstract
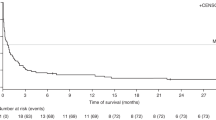
EBV-associated post-transplant lymphoproliferative disease (PTLD) following Alemtuzumab-based allo-SCT is a relatively uncommon and challenging clinical problem but has not received detailed study in a large cohort. Quantitative-PCR (qPCR) monitoring for EBV reactivation post allo-SCT is now commonplace but its diagnostic and predictive value remains unclear. Sixty-nine patients with PTLD following Alemtuzumab-based allo-SCT were studied. Marked clinicopathological heterogeneity was evident; lymphadenopathy was frequently absent, whereas advanced extranodal disease was common. The median viral load at clinical presentation was 49 300 copies/mL (50–65 200 000 copies/mL) and, notably, 23% and 45% of cases, respectively, had ⩽10 000 and ⩽40 000 copies/mL. The overall response rate to rituximab as first-line therapy was 70%. For rituximab failures, chemotherapy was ineffectual but DLIs were successful. A four-parameter prognostic index predicted response to therapy (OR 0.30 (0.12–0.74); P=0.009] and PTLD mortality (hazard ratio (HR) 1.81 (1.12–2.93) P=0.02) on multivariate analysis. This is the largest detailed series of EBV-associated PTLD after allo-SCT. At clinical presentation, EBV-qPCR values are frequently below customary thresholds for pre-emptive therapy, challenging current paradigms for monitoring and intervention. A four-point score identifies a proportion of patients at risk of rituximab-refractory disease for whom alternative therapy is needed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115: 925–935.
Landgren O, Gilbert ES, Rizzo JD, Socie G, Banks PM, Sobocinski KA et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood 2009; 113: 4992–5001.
Hale G, Waldmann H . Risks of developing Epstein-Barr virus-related lymphoproliferative disorders after T-cell-depleted marrow transplants. CAMPATH Users. Blood 1998; 91: 3079–3083.
Kottaridis PD, Milligan DW, Chopra R, Chakraverty RK, Chakrabarti S, Robinson S et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood 2000; 96: 2419–2425.
Hale G, Cobbold S, Novitzky N, Bunjes D, Willemze R, Prentice HG et al. CAMPATH-1 antibodies in stem-cell transplantation. Cytotherapy 2001; 3: 145–164.
Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G . Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy 2001; 3: 261–267.
Chakrabarti S, Milligan DW, Pillay D, Mackinnon S, Holder K, Kaur N et al. Reconstitution of the Epstein-Barr virus-specific cytotoxic T-lymphocyte response following T-cell-depleted myeloablative and nonmyeloablative allogeneic stem cell transplantation. Blood 2003; 102: 839–842.
Peggs KS, Banerjee L, Thomson K, Mackinnon S . Post transplant lymphoproliferative disorders following reduced intensity conditioning with in vivo T cell depletion. Bone Marrow Transplant 2003; 31: 725–726 author reply 727.
Zutter MM, Martin PJ, Sale GE, Shulman HM, Fisher L, Thomas ED et al. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood 1988; 72: 520–529.
Schubach WH, Hackman R, Neiman PE, Miller G, Thomas ED . A monoclonal immunoblastic sarcoma in donor cells bearing Epstein-Barr virus genomes following allogeneic marrow grafting for acute lymphoblastic leukemia. Blood 1982; 60: 180–187.
Heslop HE, Brenner MK, Rooney CM . Donor T cells to treat EBV-associated lymphoma. N Engl J Med 1994; 331: 679–680.
Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 1994; 330: 1185–1191.
Gross TG, Steinbuch M, DeFor T, Shapiro RS, McGlave P, Ramsay NK et al. B cell lymphoproliferative disorders following hematopoietic stem cell transplantation: risk factors, treatment and outcome. Bone Marrow Transplant 1999; 23: 251–258.
Styczynski J, Einsele H, Gil L, Ljungman P . Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis 2009; 11: 383–392.
Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 2012; 119: 2644–2656.
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 2002; 99: 4364–4369.
Meij P, van Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N et al. Impaired recovery of Epstein-Barr virus (EBV)—specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood 2003; 101: 4290–4297.
Ahmad I, Cau NV, Kwan J, Maaroufi Y, Meuleman N, Aoun M et al. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation 2009; 87: 1240–1245.
Omar H, Hagglund H, Gustafsson-Jernberg A, LeBlanc K, Mattsson J, Remberger M et al. Targeted monitoring of patients at high risk of post-transplant lymphoproliferative disease by quantitative Epstein-Barr virus polymerase chain reaction. Transpl Infect Dis 2009; 11: 393–399.
Carpenter B, Haque T, Dimopoulou M, Atkinson C, Roughton M, Grace S et al. Incidence and dynamics of Epstein-Barr virus reactivation after alemtuzumab-based conditioning for allogeneic hematopoietic stem-cell transplantation. Transplantation 2010; 90: 564–570.
Coppoletta S, Tedone E, Galano B, Soracco M, Raiola AM, Lamparelli T et al. Rituximab treatment for Epstein-Barr virus DNAemia after alternative-donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17: 901–907.
Bokhari S, Das-Gupta E, Russell N, Byrne J . Post-transplant lymphoproliferative disease following reduced intensity conditioning transplants incorporating alemtuzumab. Bone Marrow Transplant 2008; 42: 281–282.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT. guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43: 757–770.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244.
Shipp MA . Prognostic factors in aggressive non-Hodgkin's lymphoma: who has ‘high-risk’ disease? Blood 1994; 83: 1165–1173.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 4th edn World Health Organisation Press, Lyon, France, 2008.
Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood 2004; 103: 3979–3981.
Worth A, Conyers R, Cohen J, Jagani M, Chiesa R, Rao K et al. Pre-emptive rituximab based on viraemia and T cell reconstitution: a highly effective strategy for the prevention of Epstein-Barr virus-associated lymphoproliferative disease following stem cell transplantation. Br J Haematol 2011; 155: 377–385.
D'Aveni M, Aissi-Rothe L, Venard V, Salmon A, Falenga A, Decot V et al. The clinical value of concomitant Epstein Barr virus (EBV)-DNA load and specific immune reconstitution monitoring after allogeneic hematopoietic stem cell transplantation. Transpl Immunol 2011; 24: 224–232.
Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 2010; 116: 5045–5049.
Acknowledgements
CPF received research funding from Leukaemia and Lymphoma Research, UK. DB received research funding from the Wellcome Trust UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CPF has received travel grants and speaker honoraria from Roche UK. ARP has received travel grants, advisory board and speaker honoraria from Roche UK. SC has received travel grants from Roche UK.
Rights and permissions
About this article
Cite this article
Fox, C., Burns, D., Parker, A. et al. EBV-associated post-transplant lymphoproliferative disorder following in vivo T-cell-depleted allogeneic transplantation: clinical features, viral load correlates and prognostic factors in the rituximab era. Bone Marrow Transplant 49, 280–286 (2014). https://doi.org/10.1038/bmt.2013.170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.170
Keywords
This article is cited by
-
Outcomes for patients with EBV-positive PTLD post-allogeneic HCT after failure of rituximab-containing therapy
Bone Marrow Transplantation (2024)
-
Clinical characteristics and outcomes of Epstein-Barr virus viral load after allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2024)
-
Expert Consensus on the Characteristics of Patients with Epstein–Barr Virus-Positive Post-Transplant Lymphoproliferative Disease (EBV+ PTLD) for Whom Standard-Dose Chemotherapy May be Inappropriate: A Modified Delphi Study
Advances in Therapy (2023)
-
Characteristics of Epstein–Barr virus reactivation after allogeneic haematopoietic stem cell transplantation in patients with chronic active Epstein–Barr virus disease: favorable responses to rituximab
Bone Marrow Transplantation (2021)