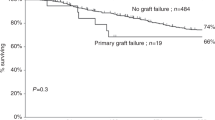
Abstract
SCID is a fatal syndrome caused by mutations in at least 13 different genes. It is characterized by the absence of T cells. Immune reconstitution can be achieved through nonablative related donor BMT. However, the first transplant may not provide sufficient immunity. In these cases, booster transplants may be helpful. A prospective/retrospective study was conducted of 49 SCID patients (28.7% of 171 SCIDs transplanted over 30 years) who had received booster transplants to define the long-term outcome, factors contributing to a need for a booster and factors that predicted success. Of the 49 patients, 31 (63%) are alive for up to 28 years. Age at initial transplantation was found to have a significant effect on outcome (mean of 194 days old for patients currently alive, versus a mean of 273 days old for those now deceased, P=0.0401). Persistent viral infection was present in most deceased booster patients. In several patients, the use of two parents as sequential donors resulted in striking T-and B-cell immune reconstitution. A majority of the patients alive today have normal or adequate T-cell function and are healthy. Nonablative booster BMT can be lifesaving for SCID.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Buckley RH . Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol 2004; 22: 625–655.
Buckley RH . Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res 2011; 49: 25–43.
Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL et al. Hematopoietic stem cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med 1999; 340: 508–516.
Myers LA, Patel DD, Puck JM, Buckley RH . Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood 2002; 99: 872–878.
Kline RM, Stiehm ER, Cowan MJ . Bone marrow ‘boosts’ following T cell depleted haploidentical bone marrow transplantation. Bone Marrow Transplant 1996; 17: 543–548.
Remberger M, Ringden O, Ljungman P, Hagglund H, Winiarski J, Lonnqvist B et al. Booster marrow or blood cells for graft failure after allogeneic bone marrow transplantation. Bone Marrow Transplant 1998; 22: 73–78.
Min CK, Kim DW, Lee JW, Min WS, Kim CC . Additional stem cell therapy for graft failure after allogeneic bone marrow transplantation. Acta Haematol 2000; 104: 185–192.
Wolff SN . Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant 2002; 29: 545–552.
Slatter MA, Bhattacharya A, Abinun M, Flood TJ, Cant AJ, Gennery AR . Outcome of boost haemopoietic stem cell transplant for decreased donor chimerism or graft dysfunction in primary immunodeficiency. Bone Marrow Transplant 2005; 35: 683–689.
Booth C, Ribeil JA, Audat F, Dal Cortivo L, Veys PA, Thrasher AJ et al. CD34 stem cell top-ups without conditioning after initial haematopoietic stem cell transplantation for correction of incomplete haematopoietic and immunological recovery in severe congenital immunodeficiencies. Br J Haematol 2006; 135: 533–537.
Al-Herz W, Bousfiha A, Casanova J-L, Chapel H, Conley ME, Cunningham-Rundles C et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency. Frontiers in Immunology 2011; 2: 54.
Chinen J, Davis J, De Ravin SS, Hay BN, Hsu AP, Linton GF et al. Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood 2007; 110: 67–73.
Buckley RH, Dees SC, O'Fallon WM . Serum immunoglobulins I. levels in normal children and in uncomplicated childhood allergy. Pediatr 1968; 41: 600–611.
Buckley RH, Dees SC . Serum immunoglobulins. III. Abnormalities associated with chronic urticaria in children. J. Allergy 1967; 40: 294–303.
Buckley RH, Schiff SE, Sampson HA, Schiff RI, Markert ML, Knutsen AP et al. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol 1986; 136: 2398–2407.
Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Cunningham-Rundles S, Dupont B et al. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood 1983; 61: 341–348.
Schiff SE, Kurtzberg J, Buckley RH . Studies of human bone marrow treated with soybean lectin and sheep erythrocytes: stepwise analysis of cell morphology, phenotype and function. Clin Exp Immunol 1987; 68: 685–693.
Railey MD, LoKhnygina Y, Buckley RH . Long term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pre-transplant chemotherapy or post-transplant GVHD prophylaxis. J Pediatr 2009; 155: 834–840.
Haddad E, Deist FL, Aucouturier P, Cavazzana-Calvo M, Blanche S, Basile GD et al. Long-term chimerism and B-cell function after bone marrow transplantation in patients with severe combined immunodeficiency with B cells: a single-center study of 22 patients. Blood 1999; 94: 2923–2930.
Buckley RH . B-cell function in severe combined immunodeficiency after stem cell or gene therapy: a review. J Allergy Clin Immunol 2010; 125: 790–797.
Buckley RH, Win CM, Moser BK, Parrott RE, Sajaroff E, Sarzotti-Kelsoe M . Post-transplantation B cell function in different molecular types of SCID. J Clin Immunol 2013; 33: 96–110.
Palmer K, Green TD, Roberts JL, Sajaroff E, Cooney M, Parrott R et al. Unusual clinical and immunologic manifestations of transplacentally acquired maternal T cells in severe combined immunodeficiency. J Allergy Clin Immunol 2007; 120: 423–428.
Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB . Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol 2012; 30: 1080–1086.
Titman P, Pink E, Skucek E, O'Hanlon K, Cole TJ, Gaspar J et al. Cognitive and behavioural abnormalities in children following haematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood 2008; 112: 3907–3913.
Sanders JE, the Seattle Marrow Transplant Group. Effects of bone marrow transplantation on reproductive function. Late Effects of Treatment for Childhood Cancer. Wiley-Liss Publishing Ltd: New York, NY, 1992 pp 95–101.
Buckley RH . The long quest for neonatal screening for severe combined immunodeficiency. J Allergy Clin Immunol 2012; 129: 597–604.
Hassan A, Booth C, Brightwell A, Allwood Z, Veys P, Rao K et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood 2012; 120: 3615–3624.
Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 2009; 360: 447–458.
Sideri A, Neokleous N, De La Grange PB, Guerton B, Le Bousse Kerdilles MC, Uzan G et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica 2011; 96: 1213–1220.
Acknowledgements
This work was supported by NIH Grants AI047605 and AI042951 and by a Dean’s Research Fellowship to CLT from Duke University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Teigland, C., Parrott, R. & Buckley, R. Long-term outcome of non-ablative booster BMT in patients with SCID. Bone Marrow Transplant 48, 1050–1055 (2013). https://doi.org/10.1038/bmt.2013.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.6