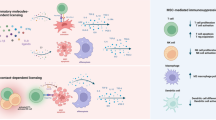
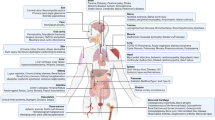
Abstract
Because of their immunomodulatory and engraftment-promoting properties, mesenchymal stromal cells (MSCs) have been tested in the clinical setting both to facilitate haematopoietic recovery and to treat steroid-resistant acute GVHD. More recently, experimental findings and clinical trials have focused on the ability of MSCs to home to damaged tissue and to produce paracrine factors with anti-inflammatory properties, resulting in functional recovery of the damaged tissue. The mechanisms through which MSCs exert their therapeutic potential rely on some key properties of the cells: the ability to secrete soluble factors capable of stimulating survival and recovery of injured cells; the capacity to home to sites of damage and the ability to blunt exaggerated immune responses. These fundamental properties are being tested within a novel therapeutic field defined as Regenerative Medicine. This review deals with recent research on the anti-inflammatory/reparative properties of MSCs and considers the possible mechanisms of function responsible for these effects. Moreover, current and potential clinical applications of MSC-based treatment strategies in the context of Regenerative Medicine are being discussed. Key issues such as optimal timing of MSC administration, cell dose and schedule of administration, advantages and disadvantages of using autologous or allogeneic cells are still open. Nonetheless, MSCs promise to represent a revolution for many severe or presently untreatable disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP . Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968; 6: 230–247.
Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA et al. Precursors for fibroblast in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 1974; 2: 83–92.
Im G-I, Shin Y-W, Lee K-B . Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage 2005; 13: 845–853.
In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004; 22: 1338–1345.
Barry FP, Murphy JM . Mesenchymal stem cells: clinical applications and biological characterization. Int J Bioch Cell Biol 2004; 36: 568–584.
Ando W, Tateishi DA, Katakai Y, Tanaka K, Nakata J, Hashimoto H et al. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials 2007; 28: 5462–5470.
Nourissat G, Diop A, Maurel N, Salvat C, Dumont S, Pigenet A et al. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS One 2010; 5: e12248.
Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001; 344: 385–386.
Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S . Autologous bone marrow stromal cell transplantation for repair of full thickness articular cartilagwe defects in human patellae: two case reports. Cell Transplant 2004; 13: 595–600.
Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a Pilot Study and Preliminary Results. Cartilage 2010; 1: 253–261.
Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 1999; 5: 309–313.
Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001; 97: 1227–1231.
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005; 106: 1755–1761.
Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol 2005; 195: 16–26.
Deng W, Han Q, Liao L, You S, Deng H, Zhao RC . Effect of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol 2005; 24: 458–463.
Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ . Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/SCID mice. Proc Natl Acad Sci USA 2006; 103: 17438–17443.
Jurewicz M, Yang S, Augello A, Godwin JG, Moore RF, Azzi J et al. Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 2010; 59: 3139–3147.
Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009; 183: 993–1004.
Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol 2006; 17: 2202–2212.
Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 2008; 26: 2075–2082.
Morigi M, Rota C, Montemurro T, Montelatici E, Lo Cicero V, Imberti B et al. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells 2010; 28: 513–522.
Hofstetter CP, Schwarz EJ, Hess D et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA 2002; 99: 2199–2204.
Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H et al. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol 2006; 199: 56–66.
Chopp M, Li Y . Treatment of neural injury with marrow stromal cells. Lancet Neurol 2002; 1: 92–100.
Pisati F, Bossolasco P, Meregalli M, Cova L, Belicchi M, Gavina M et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant 2007; 16: 41–45.
Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H et al. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther 2008; 326: 523–531.
Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 2009; 183: 7787–7798.
González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M . Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009; 136: 978–989.
Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA . Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007; 179: 1855–1863.
Lee JW, Fang X, Gupta N, Serikov V, Matthay MA . Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 2009; 106: 16357–16362.
Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One 2007; 2: e941.
van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008; 47: 1634–1643.
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD . Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002; 105: 93–98.
Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg 2002; 123: 1132–1140.
Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang Y et al. Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats. Transpl Int 2006; 19: 570–580.
Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J et al. Reversal of the immunesuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum 2005; 52: 1595–1603.
Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R . Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 2003; 101: 2999–3001.
Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004; 104: 2643–2645.
Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005; 106: 419–427.
Fox JM, Chamberlain G, Ashton BA, Middleton J . Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol 2007; 137: 491–502.
González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M . Adipose–derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009; 136: 978–989.
Kuci S, Kuci Z, Kreyenberg H, Deak E, Putsch K, Huenecke S et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica 2010; 95: 651–659.
Bernardo ME, Emons JAM, Nauta AJ, Roelofs H, Romeo S, Marchini A et al. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res 2007; 48: 132–140.
Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W . Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002; 30: 215–222.
Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol 2007; 4: 50–57.
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomorri JM, Kassis I et al. Safety and immunological effect of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010; 67: 1187–1194.
Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H et al. The therapeutich potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler 2010; 16: 503–510.
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY . A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010; 28: 1099–1106.
Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy 2009; 11: 897–911.
García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA et al. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 2005; 48: 1416–1423.
Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut (e-pub ahead of print 21 January 2011; doi:10.1136/gut.2010.214841).
Duijvenstein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut 2010; 59: 1662–1669.
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009; 54: 2277–2286.
Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 2006; 113: 1287–1294.
Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007; 110: 1362–1369.
Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol 2009; 21: 1199–1205.
Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol 2007; 13: 3359–3363.
Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med 200; 10: 459–466.
Ankrum J, Karp JM . Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med 2010; 16: 203–209.
Porada CD, Almeida-Porada G . Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev 2010; 62: 1156–1166.
Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis 2010; 69: 1423–1429.
Reinders MEJ, Fibbe WE, Rabelink TJ . Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol Dial Transplant 2010; 25: 17–24.
Matthay MA, Thompson BT, Read EJ, McKenna Jr DH, Liu KD, Calfee CS et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 2010; 138: 965–972.
Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC . Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 2009; 12: 359–366.
Lasala GP, Silva JA, Gardner PA, Minguell JJ . Combination stem cell therapy for the treatment of severe limb ischemia: safety and efficacy analysis. Angiology 2010; 61: 551–556.
Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen 2010; 18: 50–58.
Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med 2007; 2: 785–794.
Larghero J, Farge D, Braccini A, Lecourt S, Scherberich A, Foïs E et al. Phenotypical and functional characteristics of in vitro expanded bone marrow mesenchymal stem cells from patients with systemic sclerosis. Ann Rheum Dis 2008; 67: 443–449.
Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, Ceredig R et al. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology 2007; 46: 403–408.
Bernardo ME, Avanzini MA, Ciccocioppo R, Perotti C, Cometa AM, Moretta A et al. Phenotypical/functional characterization of in vitro expanded mesenchymal stromal cells from Crohn's disease patients. Cytotherapy 2009; 11: 825–836.
Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 2010; 115: 1549–1553.
Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 2008; 22: 593–599.
Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC . SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 2007; 109: 1743–1751.
Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W . Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci 2007; 1106: 262–271.
Battula VL, Treml S, Bareiss PM, Gieseke F, Roelofs H, de Zwart P et al. Isolation of functionally distinct mesenchymal stem cells subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1 (MSCA-1). Haematologica 2009; 94: 173–184.
Kubo H, Shimizu M, Taya Y, Kawamoto T, Michida M, Kaneko E et al. Identification of mesenchymal stem cell (MSC)-transcription factors by microarray and knockdown analyses, and signature molecule-marked MSC in bone marrow by immunohistochemistry. Genes to cells 2009; 14: 407–424.
Song L, Webb NE, Song Y, Tuan RS . Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells 2006; 24: 1707–1708.
Acknowledgements
This work has been partly supported by grants from Istituto Superiore di Sanità (National Program on Stem Cells), MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca, Progetti di Rilevante Interesse Nazionale, PRIN), from Associazione Italiana per la Ricerca sul Cancro (AIRC) IG9062 to MEB and by the special grant ‘5x1000’ from AIRC to FL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bernardo, M., Pagliara, D. & Locatelli, F. Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine?. Bone Marrow Transplant 47, 164–171 (2012). https://doi.org/10.1038/bmt.2011.81
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.81
Keywords
This article is cited by
-
Mesenchymal stromal cell-associated migrasomes: a new source of chemoattractant for cells of hematopoietic origin
Cell Communication and Signaling (2023)
-
Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges
Pediatric Research (2020)
-
Pharmacological tools to mobilise mesenchymal stromal cells into the blood promote bone formation after surgery
npj Regenerative Medicine (2020)
-
Immortalization of Mesenchymal Stromal Cells by TERT Affects Adenosine Metabolism and Impairs their Immunosuppressive Capacity
Stem Cell Reviews and Reports (2020)
-
Regulation of PKCβ levels and autophagy by PML is essential for high-glucose-dependent mesenchymal stem cell adipogenesis
International Journal of Obesity (2019)