Abstract
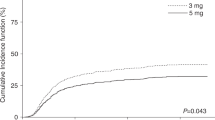
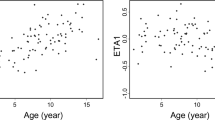
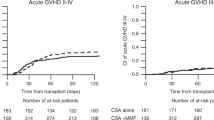
We investigated clinical factors that affected the clearance of tacrolimus (FK506) administered by continuous drip infusion to children who had received allogeneic hematopoietic SCT. Blood FK506 levels were measured every day in 27 patients in an attempt to adjust the dose to maintain the target range (10–15 ng/mL). Patients who developed engraftment syndrome (ES) and acute GVHD and patients less than 7 years of age showed a higher FK506 clearance calculated from body weight (BW) for 5 or more consecutive days compared with the control groups. A time-course study showed that the occurrence of ES, but not acute GVHD, was related to increased clearance of FK506. When calculated from body surface area (BSA), a significant increase in FK506 clearance was observed in patients with ES, but not in those less than 7 years of age. FK506 clearance was not influenced by CYP3A5, multidrug resistance 1 or ABCG2 genotypes. None of the clinical parameters affected blood FK506 levels. Determination of the FK506 dose on the basis of frequent monitoring of the blood concentration seems to minimize the serious adverse effects induced by the immunosuppressant. It may be more accurate to dose FK506 according to BSA rather than BW for pediatric patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Peters DH, Fitton A, Plosker GL, Faulds D . Tacrolimus. A review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs 1993; 46: 746–794.
Lin CS, Boltz RC, Siekierka JJ, Sigal NH . FK-506 and cyclosporin A inhibit highly similar signal transduction pathways in human T lymphocytes. Cell Immunol 1991; 133: 269–284.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998; 92: 2303–2314.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000; 96: 2062–2068.
Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant 2001; 28: 181–185.
Wingard JR, Nash RA, Przepiorka D, Klein JL, Weisdorf DJ, Fay JW et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant 1998; 4: 157–163.
Przepiorka D, Devine S, Fay J, Uberti J, Wingard J . Practical considerations in the use of tacrolimus for allogeneic marrow transplantation. Bone Marrow Transplant 1999; 24: 1053–1056.
Yanik G, Levine JE, Ratanatharathorn V, Dunn R, Ferrara J, Hutchinson RJ . Tacrolimus (FK506) and methotrexate as prophylaxis for acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Bone Marrow Transplant 2000; 26: 161–167.
Przepiorka D, Blamble D, Hilsenbeck S, Danielson M, Krance R, Chan KW . Tacrolimus clearance is age-dependent within the pediatric population. Bone Marrow Transplant 2000; 26: 601–605.
Mehta P, Beltz S, Kedar A, Graham-Pole J, Wingard JR . Increased clearance of tacrolimus in children: need for higher doses and earlier initiation prior to bone marrow transplantation. Bone Marrow Transplant 1999; 24: 1323–1327.
Yamauchi A, Ieiri I, Kataoka Y, Tanabe M, Nishizaki T, Oishi R et al. Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation 2002; 74: 571–572.
Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant 2003; 3: 477–483.
Masuda S, Inui K . An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther 2006; 112: 184–198.
Fukudo M, Yano I, Yoshimura A, Masuda S, Uesugi M, Hosohata K et al. Impact of MDR1 and CYP3A5 on the oral clearance of tacrolimus and tacrolimus-related renal dysfunction in adult living-donor liver transplant patients. Pharmacogenet Genomics 2008; 18: 413–423.
Nakazawa Y, Saito S, Hasegawa Y, Yanagisawa R, Sakashita K, Kamijo T et al. A possible role for the production of multiple HLA antibodies in fatal platelet transfusion refractoriness after peripheral blood progenitor cell transplantation from the mother in a patient with relapsed leukemia. Transfusion 2007; 47: 326–334.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Yanagisawa R, Nakazawa Y, Sakashita K, Tanaka M, Shikama N, Kamijo T et al. Low toxicity of a conditioning with 8-Gy total body irradiation, fludarabine and cyclophosphamide as preparative regimen for allogeneic hematopoietic stem cell transplantation in pediatric hematological malignancies. Pediatr Transplant 2009; 13: 737–745.
Cogill JL, Taylor PJ, Westley IS, Morris RG, Lynch SV, Johnson AG . Evaluation of the tacrolimus II microparticle enzyme immunoassay (MEIA II) in liver and renal transplant recipients. Clin Chem 1998; 44: 1942–1946.
Yamazaki T, Masumoto J, Agematsu K, Sawai N, Kobayashi S, Shigemura T et al. Anakinra improves sensory deafness in a Japanese patient with Muckle-Wells syndrome, possibly by inhibiting the cryopyrin inflammasome. Arthritis Rheum 2008; 58: 864–868.
Miura M, Kagaya H, Satoh S, Inoue K, Saito M, Habuchi T et al. Influence of drug transporters and UGT polymorphisms on pharmacokinetics of phenolic glucuronide metabolite of mycophenolic acid in Japanese renal transplant recipients. Ther Drug Monit 2008; 30: 559–564.
Boswell GW, Bekersky I, Fay J, Wingard J, Antin J, Weisdorf D et al. Tacrolimus pharmacokinetics in BMT patients. Bone Marrow Transplant 1998; 21: 23–28.
Wingard JR, Nash RA, Ratanatharathorn V, Fay JW, Klein JL, Przepiorka D et al. Lack of interaction between tacrolimus (FK506) and methotrexate in bone marrow transplant recipients. Bone Marrow Transplant 1997; 20: 49–51.
Spitzer TR . Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27: 893–898.
Tocci MJ, Matkovich DA, Collier KA, Kwok P, Dumont F, Lin S et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol 1989; 143: 718–726.
Fardel O, Lecureur V, Guillouzo A . Regulation by dexamethasone of P-glycoprotein expression in cultured rat hepatocytes. FEBS Lett 1993; 327: 189–193.
Demeule M, Jodoin J, Beaulieu E, Brossard M, Béliveau R . Dexamethasone modulation of multidrug transporters in normal tissues. FEBS Lett 1999; 442: 208–214.
Krusekopf S, Roots I, Kleeberg U . Differential drug-induced mRNA expression of human CYP3A4 compared to CYP3A5, CYP3A7 and CYP3A43. Eur J Pharmacol 2003; 466: 7–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yanagisawa, R., Katsuyama, Y., Shigemura, T. et al. Engraftment syndrome, but not acute GVHD, younger age, CYP3A5 or MDR1 polymorphisms, increases tacrolimus clearance in pediatric hematopoietic SCT. Bone Marrow Transplant 46, 90–97 (2011). https://doi.org/10.1038/bmt.2010.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.64
Keywords
This article is cited by
-
Engraftment syndrome after allogeneic stem cell transplantation: a systematic review and meta-analysis
Bone Marrow Transplantation (2023)
-
Intrapatient variability in concentration/dose ratio of tacrolimus predicts transplant-associated thrombotic microangiopathy
International Journal of Hematology (2021)
-
Safety, tolerability, and feasibility of antifungal prophylaxis with micafungin at 2 mg/kg daily in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation
Infection (2014)
-
Twice daily i.v. bolus tacrolimus infusion for GVHD prophylaxis in children undergoing stem cell transplantation
Bone Marrow Transplantation (2012)