Abstract
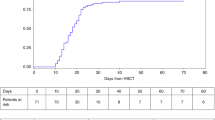
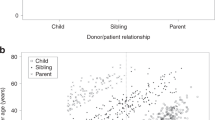
Hurler's syndrome (HS), the most severe form of mucopolysaccharidosis type-I, causes progressive deterioration of the central nervous system and death in childhood. Allogeneic stem cell transplantation (SCT) before the age of 2 years halts disease progression. Graft failure limits the success of SCT. We analyzed data on HS patients transplanted in Europe to identify the risk factors for graft failure. We compared outcomes in 146 HS patients transplanted with various conditioning regimens and grafts. Patients were transplanted between 1994 and 2004 and registered to the European Blood and Marrow Transplantation database. Risk factor analysis was performed using logistic regression. ‘Survival’ and ‘alive and engrafted’-rate after first SCT was 85 and 56%, respectively. In multivariable analysis, T-cell depletion (odds ratio (OR) 0.18; 95% confidence interval (CI) 0.04–0.71; P=0.02) and reduced-intensity conditioning (OR 0.08; 95% CI 0.02–0.39; P=0.002) were the risk factors for graft failure. Busulfan targeting protected against graft failure (OR 5.76; 95% CI 1.20–27.54; P=0.028). No difference was noted between cell sources used (bone marrow, peripheral blood stem cells or cord blood (CB)); however, significantly more patients who received CB transplants had full-donor chimerism (OR 9.31; 95% CI 1.06–82.03; P=0.044). These outcomes may impact the safety/efficacy of SCT for ‘inborn-errors of metabolism’ at large. CB increased the likelihood of sustained engraftment associated with normal enzyme levels and could therefore be considered as a preferential cell source in SCT for ‘inborn errors of metabolism’.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Neufeld EF, Muenzer J . The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New York, 2001, pp 3421–3452.
Braunlin EA, Rose AG, Hopwood JJ, Candel RD, Krivit W . Coronary artery patency following long-term successful engraftment 14 years after bone marrow transplantation in the Hurler syndrome. Am J Cardiol 2001; 88: 1075–1088.
Hobbs JR, Hugh-Jones K, Barrett AJ, Byrom N, Chambers D, Henry K et al. Reversal of clinical features of Hurler's disease and biochemical improvement after treatment by bone-marrow transplantation. Lancet 1981; 2: 709–712.
Summers CG, Purple RL, Krivit W, Pineda R, Copland GT, Ramsay NKC et al. Ocular changes in the mucopolysaccharidoses after bone-marrow transplantation. Ophthalmology 1989; 96: 977–984.
Vinallonga X, Sanz N, Balaguer A, Miro L, Ortega JJ, Casaldaliga J . Hypertrophic cardiomyopathy in mucopolysaccharidoses – regression after bone-marrow transplantation. Pediatr Cardiol 1992; 13: 107–109.
Krivit W, Henslee-Downey J, Klemperer M . Survival in Hurler's disease following bone marrow transplantation in 84 patients. Bone Marrow Transplant 1995; 15: S182–S185.
Shapiro EG, Lockman LA, Balthazor M, Krivit W . Neuropsychological outcomes of several storage diseases with and without bone-marrow transplantation. J Inherited Metab Dis 1995; 18: 413–429.
Vellodi A, Young EP, Cooper A, Wraith JE, Winchester B, Meaney C et al. Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch Dis Childhood 1997; 76: 92–99.
Peters C, Balthazor M, Shapiro EG, King RJ, Kollman C, Hegland JD et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood 1996; 87: 4894–4902.
Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ et al. Hurler syndrome: II. Outcome of HLA genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. Blood 1998; 91: 2601–2608.
Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F et al. Outcome of 27 patients with Hurler's syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant 2003; 31: 1105–1117.
Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med 2004; 350: 1960–1969.
Kurtzberg J, Krivit W . Cord blood transplantation for lysosomal storage diseases demonstrates the potential of cord blood cells for future cellular therapies. Blood (ASH meeting) 2004; 3602 (abstract).
Peters C, Shapiro EG, Krivit W . Hurler syndrome: past, present, and future. J Pediatr 1998; 133: 7–9.
Orchard PJ, Grewal S, Milla C, Braunlin EA, Defor T, Panoskaltsis-Mortari A et al. Pulmonary risk factors in allogeneic transplantation for Hurler syndrome. Blood 2004; 104: 592A.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Reiss U, Cowan M, McMillan A, Horn B . Hepatic venoocclusive disease in blood and bone marrow transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. J Pediatr Hematol Oncol 2002; 24: 746–750.
Martin P, Carter S, Kernan N, Sahdev I, Wall D, Pietryga D et al. Results of the Cord Blood Transplantation Study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant 2006; 12: 184–194.
Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med 2005; 352: 2069–2081.
Guffon N, Fouilhoux A, Bertrand Y, Thomalla M . Longitudinal neurocognitive follow-up of 26 MPSI patients with human stem cell transplantation or with enzyme replacement therapy. Ninth International Symposium on Mucopolysaccharide and Related Diseases, Venice, 2006, p 166.
Rubinstein P, Dobrila L, Rosenfield RE . Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA 1995; 92: 10119–10122.
Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 2004; 200: 123–135.
Chen N, Hudson JE, Walczak P, Misiuta I, Garbuzova-Davis S, Jiang L et al. Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural. Stem Cells 2005; 23: 1560–1570.
Meier C, Middelanis J, Wasielewski B, Neuhoff S, Roth-Haerer A, Gantert M et al. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr Res 2006; 59: 244–249.
Le Blanc K, Ringden O . Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11: 321–334.
Ryan JM, Barry FP, Murphy JM, Mahon BP . Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (London) 2005; 2: 8.
Ballen KK . New trends in umbilical cord blood transplantation. Blood 2005; 105: 3786–3792.
Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol 2004; 32: 397–407.
Wagner JE, Barker JN, Defor TE, Baker KS, Blazar BR, Eide C et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 2002; 100: 1611–1618.
Chao NJ, Emerson SG, Weinberg KI . Stem cell transplantation (cord blood transplants). Hematol ASH Educ. Program 2004, 354–371.
Bolinger AM, Zangwill AB, Slattery JT, Glidden D, DeSantes K, Heyn L et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant 2000; 25: 925–930.
Zwaveling J, Bredius RGM, Cremers SCLM, Ball LM, Lankester AC, Teepe-Twiss IM et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant 2005; 35: 17–23.
Vassal G, Koscielny S, Challine D, ValteauCouanet D, Boland I, Deroussent A et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1996; 37: 247–253.
Bolinger AM, Zangwill AB, Slattery JT, DeSantes K, Heyn L, Risler LJ et al. Target dose adjustment of busulfan using pharmacokinetic parameters in pediatric patients undergoing bone marrow transplantation for malignancy or inborn errors. Blood 1997; 90: 1665.
Baxter MA, Wynn RF, Schyma L, Holmes DK, Wraith JE, Fairbairn LJ et al. Marrow stromal cells from patients affected by MPS I differentially support haematopoietic progenitor cell development. J Inherited Metab Dis 2005; 28: 1045–1053.
Resnick IB, Shapira MY, Slavin S . Nonmyeloablative stem cell transplantation and cell therapy for malignant and non-malignant diseases. Transplant Immunol 2005; 14: 207–219.
Slavin S, Aker M, Shapira MY, Resnick I, Bitan M, Or R . Reduced-intensity conditioning for the treatment of malignant and life-threatening non-malignant disorders. Clin Transplant 2003; 26: 275–282.
Jacobsohn DA, Duerst R, Tse W, Kletzel M . Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. Lancet 2004; 364: 156–162.
Grewal SS, Krivit W, Defor TE, Shapiro EG, Orchard PJ, Abel SL et al. Outcome of second hematopoietic cell transplantation in Hurler syndrome. Bone Marrow Transplant 2002; 29: 491–496.
Acknowledgements
We thank our collaborators for sharing/providing patient information: Drs Pierre Bordigoni and Alexandra Salmon (Nancy, France), Mary Coussons (Data manager Manchester, UK), Anne Gahan (Data manager, Dublin, Ireland), Isabelle Hirsch (Data manager, Necker Hospital, Paris), Dr Claudia Haase (Jena, Germany), Susanne Matthes-Martin (Vienna, Austia), Tayfun Guengoer (Zurich, Switserland), Robbert Bredius (Leiden, the Netherlands), Stefania Varotto (Padova, Italy) and Vicky Borbon (Ghent, Belgium).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boelens, J., Wynn, R., O'Meara, A. et al. Outcomes of hematopoietic stem cell transplantation for Hurler's syndrome in Europe: a risk factor analysis for graft failure. Bone Marrow Transplant 40, 225–233 (2007). https://doi.org/10.1038/sj.bmt.1705718
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705718
Keywords
This article is cited by
-
Long term follow-up after haematopoietic stem cell transplantation for mucopolysaccharidosis type I-H: a retrospective study of 51 patients
Bone Marrow Transplantation (2023)
-
B-cell depletion abrogates immune mediated cytopenia and rejection of cord blood transplantation in Hurler syndrome
Bone Marrow Transplantation (2022)
-
Allogeneic hematopoietic stem cell transplantation for inherited metabolic disorders
International Journal of Hematology (2022)
-
Recent trends in mucopolysaccharidosis research
Journal of Human Genetics (2019)
-
Recommendations for the management of MPS IVA: systematic evidence- and consensus-based guidance
Orphanet Journal of Rare Diseases (2019)