Summary:
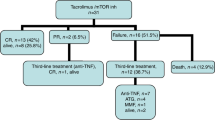
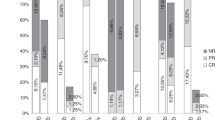
We evaluated the effect of alefacept (Amevive), a novel dimeric fusion protein, in steroid resistant/dependent acute graft-versus-host-disease (aGVHD). Seven patients were treated in eight aGVHD episodes. GVHD grade at treatment initiation and at peak ranged 2–4 (median 2.5) and 2–4 (median 4), respectively. System involvement at GVHD peak included skin (n=7), gastrointestinal tract (n=5) and liver (n=3). All patients responded. However, one patient with skin GVHD and two with gastrointestinal GVHD featuring an early initial response (IR) exacerbated and CR was not achieved. Skin GVHD responded rapidly with a median of 1 day to IR and 7 days to CR. Intestinal response was slower with median 7.5 days to IR. Of the four patients that achieved IR, CR was achieved in only one (40 days to CR). None of the patients had significant hepatic GVHD before treatment so no hepatic effect of alefacept could be determined. No immediate alefacept-related side effects were observed. Late side effects included infections (aspergillus sinusitis, pneumonia, bacteremia, pharyngeal thrush), pancytopenia and hemorrhagic cystitis. Three patients had CMV reactivation while on alefacept. We conclude that alefacept may have a beneficial effect in controlling aGVHD. Further investigations in larger cohorts of patients and controlled studies are warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lebwohl M, Christophers E, Langley R et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003; 139: 719–727.
Ellis CN, Krueger GG, Alefacept Clinical Study Group. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med 2001; 345: 248–255.
Martin PJ, Schoch G, Fisher L et al. A retrospective analysis of therapy for acute graft versus host disease: initial treatment. Blood 1990; 76: 1464–1472.
Weisdorf D, Haake R, Blazar B et al. Treatment of moderate/severe acute graft versus host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood 1990; 75: 1024–1030.
Martino R, Romero P, Subira M et al. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant 1999; 24: 283–287.
Pugatsch T, Oppenheim A, Slavin S . Improved single-step PCR assay for sex identification post-allogeneic sex-mismatched BMT. Bone Marrow Transplant 1996; 17: 273–275.
Nakamura Y, Leppert O, O’Connel P et al. Variable number of tandem repeats (VNTR) markets for human gene mapping. Science 1987; 235: 1616–1622.
Shlomchik WD, Couzens MS, Tang CB et al. Prevention of graft-versus-host disease by inactivation of host antigen-presenting cells. Science 1999; 285: 412–415.
Chatenoud L . Monoclonal antibody-based strategies in autoimmunity and transplantation. Methods Mol Med 2005; 109: 297–328.
Zeiser R, Marks R, Bertz H, Finke J . Immunopathogenesis of acute graft-versus-host disease: implications for novel preventive and therapeutic strategies. Ann Hematol 2004; 83: 551–565.
Gratama JW, Jansen J, Lipovich RA et al. Treatment of acute graft-versus-host disease with monoclonal antibody OKT3. Clinical results and effect on circulating T lymphocytes. Transplantation 1984; 38: 469–474.
Chena HR, Jia SQ, Wanga HX et al. Humanized anti-CD25 monoclonal antibody for prophylaxis of graft-vs-host disease (GVHD) in haploidentical bone marrow transplantation without ex vivo T-cell depletion. Exp Hematol 2003; 31: 1019–1025.
Morris EC, Rebello P, Thomson KJ et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood 2003; 102: 404–406.
Jacobsohn DA . Novel therapeutics for the treatment of graft-versus-host disease. Expert Opin Investig Drugs 2002; 11: 1271–1280.
Antin JH, Weisdorf D, Neuberg D et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood 2002; 100: 3479–3482.
Andolina M, Rabusin M, Maximova N, Di Leo G . Etanercept in graft-versus-host disease. Bone Marrow Transplant 2000; 26: 929.
Bashir SJ, Maibach HI . Alefacept (Biogen). Curr Opin Investig Drugs 2001; 2: 631–634.
Kaplon RJ, Hochman PS, Michler RE et al. Short course single agent therapy with an LFA-3-IgG1 fusion protein prolongs primate cardiac allograft survival. Transplantation 1996; 61: 356–363.
Krueger GG, Papp KA, Stough DB et al. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 2002; 47: 821–833.
Lebwohl M, Christophers E, Langley R et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003; 139: 719–727.
Przepiorka D, Phillips GL, Ratanatharathorn V et al. A phase II study of BTI-322, a monoclonal anti-CD2 antibody, for treatment of steroid-resistant acute graft-versus-host disease. Blood 1998; 92: 4066–4071.
Cooper JC, Morgan G, Harding S et al. Alefacept selectively promotes NK cell-mediated deletion of CD45R0+ human T cells. Eur J Immunol 2003; 33: 666–675.
Chamian F, Lowes MA, Lin SL et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci USA 2005; 102: 2075–2080.
Acknowledgements
We thank the Danny Cunniff Leukemia Research Laboratory; the Gabrielle Rich Leukemia Research Foundation; the Cancer Treatment Research Foundation; the Novotny Trust; the Szydlowsky Foundation; the Fig Tree Foundation; Ronne & Donald Hess for their continuous support of our ongoing basic and clinical research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shapira, M., Resnick, I., Bitan, M. et al. Rapid response to alefacept given to patients with steroid resistant or steroid dependent acute graft-versus-host disease: a preliminary report. Bone Marrow Transplant 36, 1097–1101 (2005). https://doi.org/10.1038/sj.bmt.1705185
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705185
Keywords
This article is cited by
-
Activation and regulation of alloreactive T cell immunity in solid organ transplantation
Nature Reviews Nephrology (2022)
-
EBMT−NIH−CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment
Bone Marrow Transplantation (2018)
-
The continuing evolution of targeted therapy for inflammatory skin disease
Seminars in Immunopathology (2016)
-
Alefacept treatment for refractory chronic extensive GVHD
Bone Marrow Transplantation (2009)