Summary:
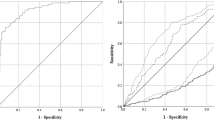
The aim of the study was to determine the prognostic value of a double primer PCR assay to detect human cytomegalovirus (HCMV) infection or disease in bone marrow transplant (BMT) recipients. A total of 209 blood samples including peripheral blood mononuclear cells (PBMN), polymorphonuclear (PMN) leukocytes and plasma from 26 BMT recipients were tested by PCR assay. To discriminate between latent and active HCMV infection, 177 blood samples were also tested by a quantitative antigenemia assay. HCMV serology status of donors and recipients was determined before transplantation by an enzyme immunosorbent assay method. Using the double primer PCR assay, the number of positive samples increased by an average of 11.6%. Symptomatic active HCMV infection was diagnosed in 14 (53.8%) out of 26 BMT patients. There was a good association between double primer PCR assay of PMN leukocytes and antigenemia assays for detection of active HCMV infection in all patients. Detection of HCMV DNA in PMN leukocytes of BMT patients by double primer PCR assay can be an alternative method for antigenemia assay. However, quantitative PCR methods will be necessary for monitoring antiviral treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ochs L, Ou Shu X, Miller J et al. Late infections after allogeneic bone marrow transplant: comparison of incidence in related and unrelated donor transplant recipients. Blood 1995; 86: 3979–3986.
Maltezou HC, Kafetzis DA, Abisaid D et al. Viral infections in children undergoing hematopoietic stem cell transplant. J Pediatr Infect Dis 2000; 19: 307–312.
Pass RF . Cytomegalovirus. In: Knipp DM, Howley PM (eds). Fields Virology, 4th edn. Lippincott-Williams & Wilkins, 2001, pp 2675–2706.
Van Der Bij W, Schirm J, Torensma R et al. Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J Clin Micorbiol 1998; 26: 2531–2535.
Cope AV, Sweny P, Sabin C et al. Quantity of cytomegalovirus viruria is a major risk factor for cytomegalovirus disease after renal transplantation. J Med Virol 1997; 52: 200–205.
Van Den Berg AP, Klompmaker IJ, Haagsma EB et al. Antigenemia in the diagnosis and monitoring of active cytomegalovirus infection after liver transplantation. J Infect Dis 1991; 164: 265–270.
Boeckh M, Hawkins G, Myerson D et al. Plasma PCR for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with PCR using peripheral blood leukocytes, pp65 antigenemia, and viral culture. Transplantation 1997; 64: 108–113.
Visser CE, Van zeijl CJG, De klerk EPA et al. First experiences with an accelerated CMV antigenemia test: CMV Brite Turbo assay. J Clin Virol 2000; 17: 65–68.
Marianne L-V, Marie O, Richard D et al. Monitoring cytomegalovirus infection in adult and pediatric bone marrow transplant recipients by a real-time PCR assay performed with blood plasma marianne. J Clin Microbiol 2003; 41: 2040–2046.
Erice A, Holm MA, Gill PC et al. Cytomegalovirus (CMV) antigenemia assay is more sensitive than shell vial cultures for rapid detection of CMV in polymorphonuclear blood leukocytes. J Clin Microbiol 1992; 30: 2822–2825.
Van der Bij W, Torensma R, Van son WJ et al. Rapid immunodiagnosis of active cytomegalovirus infection by monoclonal antibody staining of blood leucocytes. J Med Virol 1988; 25: 179–188.
Caballero OL, Menezes CL, Costa MC et al. Highly sensitive single-step PCR protocol for diagnosis and monitoring of human cytomegalovirus infection in renal transplant recipients. J Clin Microbiol 1997; 35: 3192–3197.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Stephen KN, Ho Chi-Yuen Lo, Ignatius KPC et al. Rapid cytomegalovirus pp65 antigenemia assay by direct erythrocyte lysis and immunofluorescence staining. J Clin Microbiol 1998; 36: 638–640.
Sambrook JK, Russell DW, Irwin N et al. Molecular Cloning. A Laboratory Manual, Vol. 3, 3rd edn. Cold Spring Harbor Laboratory Press: New York, 2001, p A1.23.
Saiki RK, Gelfand DH, Stoffel S et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239: 487–491.
Yakushiji K, Gondo H, Kamezaki K et al. Monitoring of cytomegalovirus reactivation after allogeneic stem cell transplantation: comparison of an antigenemia assay and quantitative real-time polymerase chain reaction. Bone Marrow Transplant 2002; 29: 599–606.
Yun Z, Lewensohn FI, Ljungman P et al. Real time monitoring of cytomegalovirus infections after stem cell transplantation using TaqMan polymerase chain reaction assays. Transplantation 2000; 27: 1733–1736.
Abecassis MM, Koffron AJ, Kaplan B et al. The role of PCR in the diagnosis and management of CMV in solid organ recipients. Transplantation 1997; 63: 275–279.
Tanabe K, Tokumoto T, Ishikawa N et al. Comparative study of cytomegalovirus (CMV) antigenemia assay, polymerase chain reaction, serology, and shell vial assay in the early diagnosis and monitoring of CMV infection after renal transplantation. Transplantation 1997; 27: 1721–1725.
Roberts TC, Brennan DC, Buller RS et al. Quantitative polymerase chain reaction to predict occurrence of symptomatic cytomegalovirus infection and assess response to ganciclovir therapy in renal transplant recipients. J Infect Dis 1998; 178: 626–635.
Acknowledgements
This project was financially supported by Tarbiat Moddares University, Tehran Iran and Shiraz University of Medical Sciences, Shiraz, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yaghobi, R., Behzad-Behbahani, A., Sabahi, F. et al. Comparative analysis of a double primer PCR assay with plasma, leukocytes and antigenemia for diagnosis of active human cytomegalovirus infection in bone marrow transplant patients. Bone Marrow Transplant 35, 595–599 (2005). https://doi.org/10.1038/sj.bmt.1704797
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704797
Keywords
This article is cited by
-
Altered Signatures of Plasma Inflammatory Proteins and Phonotypic Markers of NK Cells in Kidney Transplant Patients upon CMV Reactivation
Current Microbiology (2023)
-
Association of the costimulatory molecule gene polymorphisms and active cytomegalovirus infection in hematopoietic stem cell transplant patients
Molecular Biology Reports (2013)
-
The prevalence of molecular and immunologic infective markers of hepatitis viruses in patients with hematological malignancies
Molecular Biology Reports (2012)