Abstract
Summary:
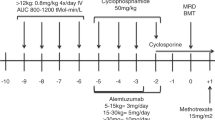
Patients with sickle cell disease (N=3) and thalassemia (N=1) with high-risk features received hematopoietic stem cell transplantations (HCT) to induce stable (full or partial) donor engraftment. Patients were 9–30 years of age. Fludarabine, rabbit anti-thymocyte globulin (ATG), and 200 cGy total body irradiation were administered pre-transplant. Patients received bone marrow (N=3) or peripheral blood stem cells (N=1) from HLA-identical siblings, followed by mycophenolate mofetil and cyclosporine for post-grafting immunosuppression. Significant lymphopenia, but only moderate neutropenia and thrombocytopenia developed post transplant. No grade IV nonhematological toxicities or acute graft-versus-host disease (GVHD) were observed. At 3 months after transplantation, three of four patients had evidence of donor myeloid chimerism (range, 15–100%). However, after post transplant immunosuppression was discontinued, graft rejection occurred in all but one patient. This patient is now doing well 27 months post transplant with full donor engraftment. One patient died after a second transplant, and another patient experienced a stroke as her graft was being rejected. These results suggest that stable donor engraftment after nonmyeloablative HCT is difficult to achieve among immunocompetent patients with hemoglobinopathies and that new approaches will need to be developed before wider application of this transplantation method for hemoglobinopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Walters MC, Storb R, Patience M et al. Impact of bone marrow transplantation for symptomatic sickle cell disease: an interim report. Blood 2000; 95: 1918–1924.
Vermylen C, Cornu G, Ferster A et al. Haematopoietic stem cell transplantation for sickle cell anaemia: the first 50 patients transplanted in Belgium. Bone Marrow Transplant 1998; 22: 1–6.
Bernaudin F, Souillet G, Vannier J . Report of French experience concerning 26 children transplanted for severe sickle cell disease. Bone Marrow Transplant 1997; 19 (Suppl 2): 112–115.
Bernaudin F . Results and current indications of bone marrow allograft in sickle cell disease. Pathol Biol (Paris) 1999; 47: 59–64.
Bernaudin F, Vernant J, Vilmer E et al. Results of myeloablative allogeneic stem cell transplant for severe sickle cell disease in France. Blood 2002; 100: 1a.
Lucarelli G, Galimberti M, Polchi P et al Bone marrow transplantation in patients with thalassemia. N Engl J Med 1990; 322: 417–421.
Lucarelli G, Clift RA, Galimberti M et al. Marrow transplantation for patients with thalassemia: results in class 3 patients. Blood 1996; 87: 2082–2088.
Lucarelli G, Clift RA, Galimberti M et al. Bone marrow transplantation in adult thalassemic patients. Blood 1999; 93: 1164–1167.
Lucarelli G, Clift R . Marrow Transplantation in Thalassemia, 3rd edn. Blackwell Scientific: Malden, MA, 2004, p 1412.
Yu C, Storb R, Mathey B et al. DLA-identical bone marrow grafts after low-dose total body irradiation: effects of high-dose corticosteroids and cyclosporine on engraftment. Blood 1995; 86: 4376–4381.
Storb R, Yu C, Wagner JL et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood 1997; 89: 3048–3054.
Sharabi Y, Sachs DH . Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med 1989; 169: 493–502.
Huang CA, Fuchimoto Y, Scheier-Dolberg R et al. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest 2000; 105: 173–181.
Andreani M, Manna M, Lucarelli G et al. Persistence of mixed chimerism in patients transplanted for the treatment of thalassemia. Blood 1996; 87: 3494–3499.
Walters MC, Blume K, Foreman S, Applebaum F (eds). Hematopoietic Cell Transplantation for Sickle Cell Disease. Blackwell Scientific: Malden, MA, 2004.
McSweeney PA, Niederwieser D, Shizuru JA et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus- tumor effects. Blood 2001; 97: 3390–3400.
Iannone R, Casella JF, Fuchs EJ et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant 2003; 9: 519–528.
Storb R, Etzioni R, Anasetti C et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood 1994; 84: 941–949.
Weinberg K, Annett G, Kashyap A et al. The effect of thymic function on immunocompetence following bone marrow transplantation. Biol Blood Marrow Transplant 1995; 1: 18–23.
Storek J, Witherspoon RP, Storb R . T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant 1995; 16: 413–425.
Preville X, Flacher M, LeMauff B et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation 2001; 71: 460–468.
Sodani P, Gaziev D, Polchi P et al. New approach for bone marrow transplantation in patients with class 3 thalassemia aged younger than 17 years. Blood 2004; 104: 1201–1203.
Fry TJ, Wayne A, Fowler D et al. Non-myeloablative allogeneic hematopoietic stem cell transplantation with pre-transplant immune depletion results in rapid full donor engraftment in pediatric patients with malignancy. Biol Blood Marrow Transplant 2004; 10 (Suppl 1): 82a.
Waller N, Langston A, Lonial S et al. Thymoglobulin pharmicokinetics and immune reconstitution in recipients of T-cell depleted CD34+ transplants from HLA mis-matched donors. Blood 2001; 98: 2807a.
Regan JF, Lyonnais C, Campbell K et al. Total and active thymoglobulin levels: effects of dose and sensitization on serum concentrations. Transpl Immunol 2001; 9: 29–36.
Bacigalupo A, Lamparelli T, Bruzzi P et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98: 2942–2947.
Storb R, Yu C, Barnett T et al. Stable mixed hematopoietic chimerism in dog leukocyte antigen-identical littermate dogs given lymph node irradiation before and pharmacologic immunosuppression after marrow transplantation. Blood 1999; 94: 1131–1136.
Maris MB, Niederwieser D, Sandmaier BM et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood 2003; 102: 2021–2030.
Yu C, Sandmaier B, Seidel K et al. Peripheral blood stem cell grafts from DLA-identical littermates result in enhanced mixed hematopoietic chimerism after non-myeloablative total body irradiation when compared to marrow grafts. Blood 1998; 92: 318b.
Acknowledgements
We are grateful for the kind and thoughtful advice provided by the advisory panel members Dorothy Moore (New Jersey Medical School) and Phyllis Bazen (Upstate Medical University and Executive Director of the Syracuse Sickle Cell Association). We also thank those advisory panel members who served on the adjudication committee: Mary Jane Petruzzi, Children's Hospital of Buffalo; Stephan Dubansky, Upstate Medical University; Norma Lerner, University of Rochester; Sharon Space, Boston University; Charles Packman, Carolinas Medical Center; Maala Varma, New Jersey Medical School. This work was supported by grants from the Genzyme Corporation, the Strong Children's Research Center and the National Institute's of Health grant no. HL 68091.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horan, J., Liesveld, J., Fenton, P. et al. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone Marrow Transplant 35, 171–177 (2005). https://doi.org/10.1038/sj.bmt.1704745
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704745
Keywords
This article is cited by
-
Durable engraftment after pharmacological pre-transplant immune suppression followed by reduced-toxicity myeloablative haploidentical stem cell transplantation in highly HLA-immunized adults with sickle cell disease
Bone Marrow Transplantation (2024)
-
Transcranial Doppler sonography and the effect of haematopoietic stem cell transplantation in sickle cell disease
Neurological Research and Practice (2022)
-
Hematopoietic stem cell transplantation for adult sickle cell disease in the era of universal donor availibility
Bone Marrow Transplantation (2018)
-
TLI-based reduced-intensity conditioning hematopoietic SCT for children and adolescents with high-risk nonmalignant disorders
Bone Marrow Transplantation (2015)
-
Hematopoietic Cell Transplantation and Sickle-Cell Disease: An Option for Everyone?
Current Pediatrics Reports (2015)