Abstract
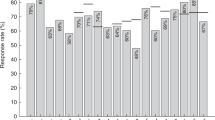
The aim of this study was to investigate the late effects of ABMT on the immune system with regard to protective humoral immunity against common antigens and responses to recall antigens (vaccines). The vaccines were given according to EBMT guidelines from 1995. The protocol included 35 patients with malignant lymphoma in CR 4–10 years after ABMT, and 35 controls. The results show that prior to ABMT the proportion of patients with protective immunity against poliomyelitis, tetanus and diphtheria was similar to that of controls. At study entry 4–10 years after ABMT, the proportion of patients with protective immunity against poliomyelitis and diphtheria was reduced, while all patients maintained protection against tetanus. A significant decrease in geometric mean antibody concentrations or titres was observed against all three antigens during this period. Serum levels of antibodies against different pneumococcal serotypes were lower in the patients than in the controls prior to vaccination. The responses to pneumococcal vaccination, which is considered to be a T cell-independent vaccine, were studied. Unlike controls, a minority of patients achieved protective levels of antibodies after a single vaccination. Despite persistent levels of protective antibodies in many patients post ABMT, secondary booster responses after one vaccination with T cell-dependent vaccines (tetanus, diphtheria and polio) were absent. In conclusion, this study shows that post ABMT, a full re-vaccination program was necessary to mount responses comparable to those observed after a single vaccination in controls. Bone Marrow Transplantation (2001) 28, 681–687.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Philip T, Guglielmi C, Hagenbeek A et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy sensitive non-Hodgkin's lymphoma New Engl J Med 1995 333: 1540–1545
Schmitz N, Sextro M, Hasenclever D et al. First results of a randomised trial comparing aggressive chemotherapy with high-dose therapy (HDT) and hematopoietic stem cell transplantation (HSCT) in patients with chemosensitive relapse of Hodgkin's disease (HD) Blood 1997 90: (Suppl. 1): Abstr. 499
Attal M, Harrousseau JL, Stoppa AM et al. A prospective randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma New Engl J Med 1996 335: 91–97
Zittoun RA, Mandelli F, Willemze R et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia New Engl J Med 1995 332: 217–223
Pedrazzini A, Freedman AS, Andersen J et al. Anti-B-cell monoclonal antibody-purged autologous bone marrow transplantation for B-cell non-Hodgkin's lymphoma; phenotypic reconstitution and B-cell function Blood 1989 74: 2203–2211
Storek J, Witherspoon RP, Storb R . T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life Bone Marrow Transplant 1995 16: 413–425
Talmadge JE, Reed E, Ino K et al. Rapid immunologic reconstitution following transplantation with mobilized peripheral blood stem cells as compared to bone marrow Bone Marrow Transplant 1997 19: 161–172
Ljungman P, Wiklund-Hammarsten M, Duraj V, et al. Response to tetanus toxoid immunization after allogenic bone marrow transplantation J Infect Dis 1990 162: 496–500
Ljungman P, Duraj V, Magnius L . Response to immunization against polio after bone marrow transplantation Bone Marrow Transplant 1991 7: 89–93
Ljungman P, Cordonnier C, de Bock R et al. Immunisations after bone marrow transplantation: results of a European survey and recommendations from the infectious diseases working party of the European Group for Blood and Marrow transplantation Bone Marrow Transplant 1995 15: 455–460
Singhal S, Metha J . Reimmunization after blood or marrow stem cell transplantation Bone Marrow Transplant 1999 23: 637–646
CDC (Centers for disease control and prevention) Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients MMMR 2000 49: No. RR–10
Nordøy T, Kolstad A, Endresen PC et al. Persistent changes in the immune system 4–10 years after ABMT Bone Marrow Transplant 1999 24: 873–878
Simonsen O, Bentzon MW, Heron I . ELIZA for the routine determination of antitoxic immunity to tetanus J Biol Stand 1987 15: 143–57
Miyamura K, Nisho S, Iti A et al. Micro cell culture method for determination of diphtheria toxin and antitoxin titres using Vero cells. I. Studies on factors affecting the toxin and antitoxin titration J Biol Stand 1974 2: 189–201
Skogen V, Jenum PA, Koreleva VN et al. Detection of diphtheria antitoxin by four different methods Clin Microbiol Infect 1999 5: 628–633
Ipsen J . Circulating antitoxin at the onset of diphtheria in 425 patients J Immunol 1946 54: 325–347
World Health Organization . Microneutralization test: Procedure. In: Guidelines for WHO/EPI collaborative studies on poliomyelitis. 1993 WHO/EPI/GEN/ 93: 5–10
World Health Organization . The immunological basis for immunization. Poliomyelitis. 1993 WHO/EPI/GEN/ 93: 16
Aaberge IS, Steinsvik TE, Groeng E-C et al. Human antibody response to a pneumococcal vaccine in SCID-PBL-hu mice and simultaneously vaccinated human cell donors Clin Exp Immunol 1996 105: 12–17
Stack AM, Malley R, Thompson CM et al. Minimum protective serum concentrations of pneumococcal anti-capsular antibodies in infant rats J Infect Dis 1998 177: 986–990
Pauksen K, Hammarstrøm V, Ljungman P et al. Immunity to poliovirus and immunization with inactivated poliovirus vaccine after autologous bone marrow transplantation Clin Infect Dis 1994 18: 547–52
Hammarstrøm V, Pauksen K, Bjørkstrand B et al. Tetanus immunity in autologous bone marrow and blood stem cell transplant recipients Bone Marrow Transplant 1998 22: 67–81
Lum LG, Munn NA, Schanfield MS et al. The detection of specific antibody formation to recall antigens after human bone marrow transplantation Blood 1986 67: 582–587
Sandbu S, Nøkleby H, Flugsrud LB . Vaksinasjon mot poliomyelitt-justeringer av anbefalt vaksinasjonsregime. MSIS rapport meldingssystem for smittsomme sykdommer. (Vaccination against poliomyelitis changes in the recommended vaccination program) Weekly Newslett Natl Inst Pub Health, Oslo, 1996 24: 39
Simonsen O, Kjeldsen K, Heron I . Immunity against tetanus and effect of revaccination 25–30 years after primary vaccination Lancet 1984 2: 1240–1242
Kjeldsen K, Simonsen O, Heron I . Immunity against diphtheria 25–30 years after primary vaccination in childhood Lancet 1985 1: 900–902
Jenum P A, Skogen V, Danilova E et al. Immunity to diphtheria in Northern Norway and Northwestern Russia Eur J Clin Microbiol Infect Dis 1995 14: 794–798
Parkkali T, Ruutu T, Stenvik M et al. Loss of protective immunity to polio, diphtheria and Haemophilus influenza type b after allogenic bone marrow transplantation APMIS 1996 104: 383–388
Roux E, Helg C, Chapuis B et al. T-cell repertoire complexity after allogeneic bone marrow transplantation: significant differences between recipients of T-cell depleted and unmanipulated grafts Blood 1996 87: 3984–3992
Roux E, Dumont-Girard F, Starobiski M et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity Blood 2000 96: 2299–2303
Talmadge JE, Singh R, Ino K et al. Mechanism of immune dysfunction in stem cell transplantation Int J Immunopharmacol 2000 1041–1056
Rassenti LZ, Kipps TJ . Immunoglobulin genes. In: Rose NR, de Macrio EC, Folds JM et al (eds) Manual and Clinical Laboratory Immunology, fifth edn ASM Press: Washington, DC 1997 p 147
Szakal AK, Kosco MH, Tew JG . Microanatomy of lymphoid tissue during the induction and maintenance of humoral immune response: structure function relationships Ann Rev Immunol 1989 7: 91–111
Zinkernagel RM, Bachmann MF, Kundig TM et al. On immunological memory Annu Rev Immunol 1996 14: 333–67
Ho F, Lortan JE, MacLennan I et al. Distinct short-lived and long-lived antibody-producing cell populations Eur J Immunol 1986 16: 1297–1301
Levy M, Vieira P, Coutinho A et al. The majority of “natural” immunoglobulin-secreting cells are short-lived and the progeny of cycling lymphocytes Eur J Immunol 1987 17: 849–854
Slifka MK, Antia R, Whitmire JK et al. Humoral immunity due to long-lived plasma cells Immunity 1998 8: 363–372
Mantz RA, Thiel A, Radbruch A . Lifetime of plasma cells in the bone marrow Nature 1997 388: 133–134
Acknowledgements
The skilful technical assistance of I Bjørnson-Langen and BW Iversen is gratefully acknowledged. We thank Kristian Bartnes and Melissa K Tuck for carefully reviewing the manuscript and Bjørn Straume for valuable statistical consultations.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nordøy, T., Husebekk, A., Aaberge, I. et al. Humoral immunity to viral and bacterial antigens in lymphoma patients 4–10 years after high-dose therapy with ABMT. Serological responses to revaccinations according to EBMT guidelines. Bone Marrow Transplant 28, 681–687 (2001). https://doi.org/10.1038/sj.bmt.1703228
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1703228
Keywords
This article is cited by
-
Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network
Leukemia (2021)
-
Fibrotic liver microenvironment promotes Dll4 and SDF-1-dependent T-cell lineage development
Cell Death & Disease (2019)
-
References
Bone Marrow Transplantation (2009)
-
Immune Deficits in Allogeneic Hematopoietic Stem Cell Transplant (HSCT) Recipients
Mycopathologia (2009)
-
Immunity for tumors and microbes after autotransplantation: if you build it, they will (not) come
Bone Marrow Transplantation (2006)