Abstract
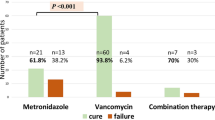
Invasive fungal infections (IFI) are increasingly diagnosed in patients undergoing allogeneic BMT. We have previously shown that the addition of metronidazole to ciprofloxacin for gastrointestinal bacterial decontamination significantly reduces the incidence of grades II–IV aGVHD by reduction of the anaerobic intestinal bacterial flora. Here, we found that the combined use of ciprofloxacin, metronidazole and fluconazole as antifungal prophylaxis increased intestinal yeast colonization when compared to ciprofloxacin and fluconazole alone (P < 0.01). Based on the EORTC criteria, a total of 18 out of 134 study patients developed IFI: seven of 68 (10%) patients who received metronidazole compared to 11 of the 66 (17%) patients decontaminated without metronidazole developed IFI (log-rank P = 0.36). Lethal IFI occurred in two of seven patients receiving metronidazole and in four of 11 patients without anaerobic decontamination. In conclusion, bacterial intestinal decontamination using metronidazole as an antibiotic with activity against most anaerobic intestinal bacteria significantly increases the intestinal yeast burden without influencing the incidence of IFI in patients undergoing allogeneic BMT. Bone Marrow Transplantation (2000) 26, 993–997.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wingard JR . Advances in the management of infectious complications after bone marrow transplantation Bone Marrow Transplant 1990 6: 371–383
Krüger WH, Kroger N, Russmann B et al. Treatment of mycotic infections after haemopoietic progenitor cell transplantation with liposomal amphotericin-B Bone Marrow Transplant 1998 22: (Suppl. 4) 10–13
Storb R, Deeg HJ, Whitehead J et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after bone marrow transplantation for leukemia New Engl J Med 1986 314: 729–735
Storb R, Deeg HJ, Pepe M et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial Blood 1989 73: 1729–1734
Atkinson K, Farrell C, Chapman G et al. Female marrow donors increase the risk of acute graft versus host disease: effect of donor age and parity and analysis of cell subpopulation in the donor inocculum Br J Haematol 1986 63: 231–239
Van Bekkum DW, Roodenburg J, Heidt PJ et al. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora J Nat Cancer Inst 1974 52: 401–404
Van Bekkum DW, Knaan S . Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction J Nat Cancer Inst 1977 58: 787–790
Gale RP, Bortin MM, van Bekkum DW et al. Risk factors for acute graft versus host disease Br J Haematol 1987 67: 397–406
Beelen DW, Elmaagacli A, Müller KD et al. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial Blood 1999 93: 3267–3275
Samonis G, Gikas A, Anaissie EJ et al. Prospective evaluation of effects of broad spectrum antibiotics on gastrointestinal yeast colonization of humans Antimicrob Agents Chemother 1993 37: 51–53
Ottinger HD, Albert E, Arnold R et al. German consensus on immunogenetic donor search for transplantation of allogeneic bone marrow and peripheral blood stem cells Bone Marrow Transplant 1997 20: 101–105
Beelen DW, Haralambie E, Brandt H et al. Evidence that sustained growth suppression of intestinal anaerobic bacteria reduces the risk of acute graft versus host disease after sibling marrow transplantation Blood 1992 80: 2668–2676
Denning DW, Marinus A, Cohen J et al. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC invasive fungal infections J Infect 1998 37: 173–180
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Trenschel, R., Peceny, R., Runde, V. et al. Fungal colonization and invasive fungal infections following allogeneic BMT using metronidazole, ciprofloxacin and fluconazole or ciprofloxacin and fluconazole as intestinal decontamination. Bone Marrow Transplant 26, 993–997 (2000). https://doi.org/10.1038/sj.bmt.1702655
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1702655
Keywords
This article is cited by
-
Influence of intestinal decontamination using metronidazole on the detection of methanogenic Archaea in bone marrow transplant recipients
Bone Marrow Transplantation (2003)