Abstract
The impact of bone marrow (BM) GD2-positive cells on survival has been evaluated in 145 Italian children with localised neuroblastoma (NB) evaluated at diagnosis by anti-GD2 immunocytochemistry. Nineteen of these (13.1%) were found to be BM GD2-positive, with the number of positive cells ranging between 1 and 155 out of 1 × 106 total cells analysed. Seven/19 (38.8%) GD2-positive vs 12/126 (9.5%) GD2-negative patients relapsed. The 5-year event-free survival (EFS) and overall survival of the GD2-positive patients was significantly worse than that of the GD2-negative ones (62.2 vs 89.9%, P<0.001; and 74.9 vs 95.9%, P=0.005, respectively). GD2 positivity was not associated to other known risk factors, and in particular to Myc-N amplification and 1p deletion. Among Myc-N-negative patients, the EFS of those negative for both GD2 and 1p deletion was significantly better than in children positive for either one of these two markers (EFS=96.9 vs 66.0%, P<0.001). In conclusion, GD2 positivity may represent a prognostic marker for patients with non-metastatic NB without Myc-N amplification, and its combination with genetic alterations might help identifying patients that require a more careful follow-up.
Similar content being viewed by others
Main
Neuroblastoma (NB) is the most common extracranial solid malignancy of childhood (Henry et al, 2005). Staging, clinical management and prognosis mainly depend on the presence/absence of bone marrow (BM) and skeletal involvement (Brodeur et al, 1993). Almost 50% of NB patients present at diagnosis with localised disease, that is, they do not have evidence of BM metastases, as assessed by morphological examination of both marrow smears and trephine biopsies, nor other distant localisations investigated by 123I-MIBG scintigraphy.
Usually patients with localised disease are treated by surgery alone (stage 1 and 2) or by standard-dose chemotherapy followed by surgery (stage 3), unless amplification of the Myc-N proto-oncogene is detected in their tumour cells (Rubie et al, 1997b; Haase et al, 1999; Simon et al, 2004; Henry et al, 2005; Maris, 2005), which requires a more aggressive chemotherapeutic regimen. Event-free (EFS) and overall (OS) survival of the patients with localised disease without Myc-N amplification are good (95% for stage 1, 86% stage 2 and 65% for stage 3 patients; Cotterill et al, 2000), but a small percentage of them relapse and may die of disease.
Genetic abnormalities at chromosome 1p (Rubie et al, 1997a), 3p, 11q (Spitz et al, 2003; Attiyeh et al, 2005; Simon et al, 2006) and 17q (Brinkschmidt et al, 2001), as well as biochemical (Simon et al, 2003), histological (Perez et al, 2000; Navarro et al, 2006; Sano et al, 2006), and biological factors (Christiansen et al, 1995; Cheung et al, 1997; Kramer et al, 1997; Perez et al, 2000; Ladenstein et al, 2001; Mora et al, 2001; Krams et al, 2003; Riley et al, 2004; Haber et al, 2006; Spitz et al, 2006), do not seem to have the same relevance in patients with localised NB as they do in patients with metastatic disease. Gene expression profiling and GCH studies have suggested specific favourable and unfavourable NB signatures (Takita et al, 2004; Ohira et al, 2005; Vandesompele et al, 2005), but presently a widespread identification of patients at risk of relapse by these techniques cannot be envisaged. Therefore, an independent, easily applicable, prognostic marker able to identify patients that would benefit from a more careful follow-up is currently lacking.
In a previous study, aimed to assess the diagnostic and prognostic role of different techniques detection of NB tumour cells in peripheral blood and BM, we observed that in patients with localised NB the GD2 positivity in BM was negatively associated with survival (Corrias et al, 2004). However, this finding was based on a small sample size with a relatively short follow-up. The aim of the present investigation was to evaluate the impact of BM GD2 positivity, evaluated at diagnosis by anti-GD2 immunocytochemistry (IC), and its combined effect with other known risk factors on survival of a larger cohort of patients with localised NB.
Materials and methods
Patients
One hundred and forty-five consecutive NB patients, diagnosed with localised disease (stages 1–3) according to INSS criteria (Brodeur et al, 1993) at 20 Italian paediatric oncology centres between January 1997 and June 2003, with available information on BM GD2 status at diagnosis, were included in the study. Disease staging (Brodeur et al, 1993) at diagnosis, including appropriate imaging, 123I MIBG scintigraphy and BM evaluation, was made at the referring oncology centre and centrally reviewed at the Gaslini Institute.
Therapeutic approach for stage 1–2 patients included only surgery followed by a complete re-evaluation with appropriate imaging 1 month later. Stage 3 patients also received chemotherapy according to national or international protocols. Stage 3 and 2 Myc-N-amplified patients received high-dose chemotherapy, myeloablative therapy with haematopoietic stem cell rescue and local radiotherapy. In case of relapse, a complete restaging, including imaging and BM evaluation, was performed.
For each patient, demographic, clinical and follow-up data, together with information on biological characteristics and other prognostic risk factors as serum LDH, NSE and ferritin, tumour Myc-N amplification and 1p status (Ambros et al, 2003), are summarised in Table 1. Data were retrieved from the Italian Neuroblastoma Registry (INBR) that collects information on clinical and biological characteristics of patients at diagnosis as treatment; follow-up is sought during protocol administration and then at least yearly after treatment discontinuation (Conte et al, 2006). Pathology data regarding the primary tumour were not considered for this study, since information according to the criteria proposed by the International Neuroblastoma Pathology Committee (INPC) was not available for patients diagnosed before 2003.
BM analysis
In general, BM aspirations and bone trephine biopsies at both iliac crests were performed under general anaesthesia during surgical procedure on the primary tumour or during ‘ad hoc’ sedation. For morphological analysis, three May Grünwald–Giemsa-stained slides from each site were examined by an experienced cytomorphologist and centrally reviewed. Trephine biopsies were obtained by a Jamshidi needle, and only biopsies containing at least 5 mm3 of tissue were considered adequate for evaluation. At least 30 high-resolution fields of haematoxylin–eosin-stained sections were evaluated by an experienced pathologist and centrally reviewed.
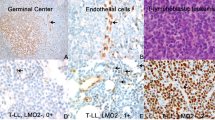
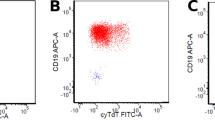
For the purpose of this study, GD2-IC was centrally performed at the Italian NB reference laboratory, as previously described (Corrias et al, 2004). Briefly, six cytospins, each containing 5 × 105 mononuclear cells, were fixed in cold acetone and incubated with the 3F8 anti-GD2 mAb (kindly donated by Dr Nai-Kong Cheung, Memorial Sloan Kettering Cancer Center, New York, NY, USA). After washing, slides were incubated with a biotinylated anti-mouse antibody and developed with an avidin–alkaline phosphatase conjugate (DAKO, Copenhagen, Denmark). Enumeration of GD2-positive cells was based on both morphological and immunological criteria, according to standardised conditions (Swerts et al, 2005). Namely, the presence of round nuclei larger than that of small lymphocytes, granular chromatic structure and scarce amount of cytoplasm were considered positive morphological criteria; strong red staining localised to the entire cell membrane and cytoplasm was the positive immunological criterion. Bone marrow samples were considered positive if at least three positive tumour cells were detected out of the 3 × 106 cells analysed.
The study was approved by the Institutions' Ethical Committees. All analyses were performed after informed consent was given from the patients themselves or their legal guardians, according to the Helsinki declaration.
Statistical analysis
Descriptive statistics were reported as percentages for categorical variables. For continuous and counting data, medians with interquartile range (IQR) were used due to the non-normal distribution of the observations and to reduce the effect of outliers. Patients were stratified according to their BM GD2 status and comparisons of frequency data were performed by means of the χ2-test or the Fisher's exact test, when appropriate. The Wilcoxon Mann–Whitney test was used to compare median values, while the Spearman ρ coefficient was used to assess correlation between variables. Event-free survival and OS analyses were performed according to the Kaplan–Meier method and compared by the log-rank test. A P-value <0.05 was considered as statistically significant. Analyses were performed using Stata for Windows statistical package (release 7.0; Stata Corporation, College Station, TX, USA).
Results
During the study period, 145 patients diagnosed with localised NB and registered in the INBR had their BM aspirates analysed at diagnosis by anti-GD2-IC. Patients included in the study were similar for age, sex, stage, Myc-N status and survival to the children with localised NB without information on GD2 status at diagnosis (see Supplementary data).
Demographic, clinical, biochemical and genetic features of the 145 study patients stratified according to BM GD2-IC status are reported in Table 1. In more detail, 126 patients (86.9%) were GD2 negative and 19 (13.1%) were GD2 positive. Among the 19 GD2-positive patients (Table 2), the number of positive cells ranged between 1 and 155 (median=3; IQR 2-20) out of 106 total cells examined. It is to be noted that of the 11 patients with less than five GD2-positive cells/106 total cells, seven were also evaluated by RT-PCR and all but one were found to be positive for at least one NB molecular marker (data not shown). Three examples of GD2-positive samples are shown in Figure 1. As reported in Table 1, no association was found between GD2 status and each of the other risk factors considered.
Seven (36.8%) of the 19 GD2-positive patients relapsed, all locally, and four of them died due to local disease progression (Table 2). The 5-year EFS and OS of this group were 62.2 and 74.9%, respectively (Table 1; Figure 2A and B). In these patients, an inverse correlation between the number of GD2-positive cells and the time to relapse was observed (ρ= −0.786, P=0.036). Conversely, among the 126 BM GD2-negative patients, only 12 (9.5%) relapsed, 10 locally and two with metastatic disease, and five subsequently died (Table 2) with a 5-year EFS of 89.9% and an OS of 95.9% (Table 1; Figure 2A and B). Differences in EFS and OS between the two groups were significant (P<0.001 and P=0.005, respectively; Table 1). If, as in our previous study, the five cells/106 cut off was used to discriminate positive BM samples, differences in survival remained highly significant (data not shown). Table 3 further reports on the 5-year EFS analyses on the entire cohort also considering other clinical and biological risk factors. A statistically significant worse effect was observed for unresectable disease (i.e., stage 3) (EFS=76.7%) vs resectable disease (i.e., stage 1–2) (EFS=89.8%; P=0.033), high LDH levels (72.1 vs 89.5%, P=0.010), Myc-N amplification (EFS=57.1 vs 90.4%, P<0.001) and chromosome 1p status (EFS=45.8% for deletion, 83% for imbalance and 92.7% for normal, P<0.001). The negative effect of 1p deletion remained even if 1p imbalance was pooled with the not deleted cases (data not shown); for this reason, in subsequent analyses patients with 1p imbalance were grouped with the not deleted ones.
(A) Event-free survival of patients with localised NB stratified by BM GD2-IC status. (B) Overall survival of patients with localised NB stratified by GD2-IC status. (C) Event-free survival of patients with normal Myc-N status stratified by GD2-IC status. (D) Event-free survival of patients with normal Myc-N status stratified by GD2-IC and chromosome 1p status.
Since Myc-N status directs patients towards either standard or high-risk treatment, survival analysis was repeated in patients with and without Myc-N amplification. In Myc-N-amplified patients, GD2 negativity was associated with a better survival (EFS 66 vs 0%), but the difference was not significant (P=0.073), likely due to the small number of cases. However, in Myc-N non-amplified patients, the BM GD2 negativity was associated with a significantly better outcome (EFS=93.2 vs 72.7%, P=0.008; Figure 2C). In the same Myc-N-negative patients, the combined prognostic role of GD2 and 1p status was also assessed. Patients negative for both markers had a 96.9% EFS, which was significantly better than that observed among children positive for at least one marker (EFS=66.0%; P<0.001; Figure 2D). In the same group of Myc-N-negative patients, the OS showed a similar pattern (98% for the GD2-negative subjects vs 83% for the GD2-positive subjects). Finally the OS of patients positive for either GD2 or Myc-N was worse than that of patients negative for both the markers (83.1 vs 100%), but the very low number of observed deaths prevents one to draw definitive conclusions.
Finally, among BM GD2-IC-positive patients, a worse survival was found in children with ⩾20 GD2-positive cells/106 total cells, corresponding to the fourth quartile (EFS=77.1 vs 20.0%, P=0.002). A similar result was also observed after excluding the two patients with Myc-N amplification (data not shown).
Because of the small sample size (19 BM GD2-positive patients) and low number of events, results from a multivariate analysis should be taken with caution and are herewith reported only as Supplementary data. In this analysis, the GD2-IC status seemed to maintain its prognostic role independently from both Myc-N amplification and 1p deletion. It is of note that conversely Myc-N amplification and 1p deletion were indeed correlated, being 72.7% of Myc-N amplified patients also deleted of 1P.
Discussion
This study has confirmed our previous observation (Corrias et al, 2004) of a negative impact of BM GD2-positive cell infiltration on survival of patients with localised NB. In particular, in this larger cohort of patients with a longer follow-up, we have shown that this effect is not due to the association with other risk factors as Myc-N amplification and 1p deletion.
Other reports have previously documented that tumour cells may be detected by IC and/or RT-PCR in BM of children with localised NB (Moss et al, 1991; Cheung et al, 1998; Trager et al, 2003; Corrias et al, 2004; Simon et al, 2004; Ifversen et al, 2005; Russell et al, 2005; Swerts et al, 2006). However, the limited number of patients analysed and/or the short follow-up of those studies did not allow one to draw definitive conclusions on the prognostic impact of this detection. In addition, a large multi-centre European study (Navarro et al, 2006) and several other national studies (Christiansen et al, 1995; Cheung et al, 1997; Ladenstein et al, 2001; Simon et al, 2003; Sano et al, 2006; Spitz et al, 2006) have indicated that stage and Myc-N status were the only independent risk factors for children with localised NB. However, Myc-N amplification is a relatively rare event, occurring, as in our series, in about 10% of localised NB patients (Haase et al, 1999; Perez et al, 2000; Henry et al, 2005; Maris, 2005) and is thus inadequate to identify all the patients who will eventually relapse.
A negative prognostic role of unfavourable histology (Perez et al, 2000), of 11q LOH (Attiyeh et al, 2005; Simon et al, 2006), and of a specific GCH profile (Schleiermacher et al, 2007), was also previously reported in patients with localised disease. This effect was independent from Myc-N status. Unfortunately, homogeneous information on tumour histology (i.e., INPC classification) was not available in our registry because of the long recruitment period covered by this study. Moreover, our patients were not screened for genetic abnormalities other than Myc-N amplification and 1p deletion. Even if genetic screening could have been more precise, our observation of a negative prognostic role of BM GD2-IC positivity suggests that also this technique, which has become accurate, thanks to recent standardisation (Swerts et al, 2005), might be of interest in future studies.
The low relapse rate observed in our cohort is similar to that expected in patients with localised NB, and indicates that staging was correct. The fact that presence of GD2-positive cells in the BM correlated with a higher tendency of relapse at the primary site, instead that in the BM, is surprising. However, also Perez et al (2000) did not observe further BM involvement in eight stage 1–2 patients found positive by BM immunocytology. Thus, it is conceivable that the very few GD2-positive cells, detected by IC at diagnosis, were unable to survive and actively proliferate in the BM. Whether this inability to invade the marrow compartment was related to the NB cells themselves or to the presence/absence of factors in the BM microenvironment (Fidler, 2003) remains to be determined in future studies.
Our observation that higher number of GD2-positive cells correlated with poorest survival and with a shorter time to relapse is similar to that previously reported by Moss et al (1991). Even if this finding was based on a very small sample size (i.e., seven relapses in GD2-positive patients), it suggests that quantification by IC might add useful clinical information and that BM GD2-IC positivity reflects a biological feature of the neuroblasts that can affect the disease course.
In our study, survival analysis on other prognostic factors confirmed the negative role of Myc-N amplification, high LDH serum levels and 1p deletion. Only a multivariable analysis based on a larger series will be able to assess the combined effect of BM GD2 positivity and the other known major risk factors. However, our data suggest that in patients with localised disease without Myc-N amplification, the combination of BM GD2-IC and 1p status might help individuating those at risk of relapse.
In conclusion, we believe that BM GD2-IC analysis might have the potential to discriminate different risk groups within localised NB patients. In fact, GD2-IC seems to provide additional information on biological features and has the advantage of generating quantitative data. We neither recommend that BM GD2-IC status be used for staging purposes, nor the patient be shifted to a more intensive treatment because not all the GD2 positive patients relapsed. However, in future multi-national studies, patients with localised disease should be evaluated by GD2-IC. In fact, in the presence of BM GD2-positive cells, especially if combined with chromosome 1p deletion, patients should be closely followed, and in case of relapse, they should be treated more aggressively.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ambros IM, Benard J, Boavida M, Bown N, Caron H, Combaret V, Couturier J, Darnfors C, Delattre O, Freeman-Edward J, Gambini C, Gross N, Hattinger CM, Luegmayr A, Lunec J, Martinsson T, Mazzocco K, Navarro S, Noguera R, O'Neill S, Potschger U, Rumpler S, Speleman F, Tonini GP, Valent A, Van Roy N, Amann G, De Bernardi B, Kogner P, Ladenstein R, Michon J, Pearson AD, Ambros PF (2003) Quality assessment of genetic markers used for therapy stratification. J Clin Oncol 21: 2077–2084
Attiyeh EF, London WB, Mosse YP, Wang Q, Winter C, Khazi D, McGrady PW, Seeger RC, Look AT, Shimada H, Brodeur GM, Cohn SL, Matthay KK, Maris JM (2005) Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med 353: 2243–2253
Brinkschmidt C, Christiansen H, Terpe HJ, Simon R, Lampert F, Boecker W, Dockhorn-Dworniczak B (2001) Distal chromosome 17 gains in neuroblastomas detected by comparative genomic hybridization (CGH) are associated with a poor clinical outcome. Med Pediatr Oncol 36: 11–13
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F (1993) Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11: 1466–1477
Cheung IY, Barber D, Cheung NK (1998) Detection of microscopic neuroblastoma in marrow by histology, immunocytology, and reverse transcription-PCR of multiple molecular markers. Clin Cancer Res 4: 2801–2805
Cheung NK, Kushner BH, LaQuaglia MP, Kramer K, Ambros PF, Ambros I, Ladanyi M, Eddy J, Bonilla MA, Gerald W (1997) Survival from non-stage 4 neuroblastoma without cytotoxic therapy: an analysis of clinical and biological markers. Eur J Cancer 33: 2117–2120
Christiansen H, Sahin K, Berthold F, Hero B, Terpe HJ, Lampert F (1995) Comparison of DNA aneuploidy, chromosome 1 abnormalities, MYCN amplification and CD44 expression as prognostic factors in neuroblastoma. Eur J Cancer 31: 541–544
Conte M, Parodi S, De Bernardi B, Milanaccio C, Mazzocco K, Angelini P, Viscardi E, Di Cataldo A, Luksch R, Haupt R (2006) Neuroblastoma in adolescents: the Italian experience. Cancer 106: 1409–1417
Corrias MV, Faulkner LB, Pistorio A, Rosanda C, Callea F, Lo Piccolo MS, Scaruffi P, Marchi C, Lacitignola L, Occhino M, Gambini C, Tonini GP, Haupt R, De Bernardi B, Pistoia V, Garaventa A (2004) Detection of neuroblastoma cells in bone marrow and peripheral blood by different techniques: accuracy and relationship with clinical features of patients. Clin Cancer Res 10: 7978–7985
Cotterill SJ, Pearson AD, Pritchard J, Foot AB, Roald B, Kohler JA, Imeson J (2000) Clinical prognostic factors in 1277 patients with neuroblastoma: results of The European Neuroblastoma Study Group ‘Survey’ 1982–1992. Eur J Cancer 36: 901–908
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3: 453–458
Haase GM, Perez C, Atkinson JB (1999) Current aspects of biology, risk assessment, and treatment of neuroblastoma. Semin Surg Oncol 16: 91–104
Haber M, Smith J, Bordow SB, Flemming C, Cohn SL, London WB, Marshall GM, Norris MD (2006) Association of high-level MRP1 expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J Clin Oncol 24: 1546–1553
Henry MC, Tashjian DB, Breuer CK (2005) Neuroblastoma update. Curr Opin Oncol 17: 19–23
Ifversen MR, Kagedal B, Christensen LD, Rechnitzer C, Petersen BL, Heilmann C (2005) Comparison of immunocytochemistry, real-time quantitative RT-PCR and flow cytometry for detection of minimal residual disease in neuroblastoma. Int J Oncol 27: 121–129
Kramer K, Cheung NK, Gerald WL, LaQuaglia M, Kushner BH, LeClerc JM, LeSauter L, Saragovi HU (1997) Correlation of MYCN amplification, Trk-A and CD44 expression with clinical stage in 250 patients with neuroblastoma. Eur J Cancer 33: 2098–2100
Krams M, Hero B, Berthold F, Parwaresch R, Harms D, Rudolph P (2003) Full-length telomerase reverse transcriptase messenger RNA is an independent prognostic factor in neuroblastoma. Am J Pathol 162: 1019–1026
Ladenstein R, Ambros IM, Potschger U, Amann G, Urban C, Fink FM, Schmitt K, Jones R, Slociak M, Schilling F, Ritter J, Berthold F, Gadner H, Ambros PF (2001) Prognostic significance of DNA di-tetraploidy in neuroblastoma. Med Pediatr Oncol 36: 83–92
Maris JM (2005) The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr 17: 7–13
Mora J, Cheung NK, Chen L, Qin J, Gerald W (2001) Survival analysis of clinical, pathologic, and genetic features in neuroblastoma presenting as locoregional disease. Cancer 91: 435–442
Moss TJ, Reynolds CP, Sather HN, Romansky SG, Hammond GD, Seeger RC (1991) Prognostic value of immunocytologic detection of bone marrow metastases in neuroblastoma. N Engl J Med 324: 219–226
Navarro S, Amann G, Beiske K, Cullinane CJ, d'Amore ES, Gambini C, Mosseri V, De Bernardi B, Michon J, Peuchmaur M (2006) Prognostic value of International Neuroblastoma Pathology Classification in localized resectable peripheral neuroblastic tumors: a histopathologic study of localized neuroblastoma European Study Group 94.01 Trial and Protocol. J Clin Oncol 24: 695–699
Ohira M, Oba S, Nakamura Y, Isogai E, Kaneko S, Nakagawa A, Hirata T, Kubo H, Goto T, Yamada S, Yoshida Y, Fuchioka M, Ishii S, Nakagawara A (2005) Expression profiling using a tumor-specific cDNA microarray predicts the prognosis of intermediate risk neuroblastomas. Cancer Cell 7: 337–350
Perez CA, Matthay KK, Atkinson JB, Seeger RC, Shimada H, Haase GM, Stram DO, Gerbing RB, Lukens JN (2000) Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: a children's cancer group study. J Clin Oncol 18: 18–26
Riley RD, Heney D, Jones DR, Sutton AJ, Lambert PC, Abrams KR, Young B, Wailoo AJ, Burchill SA (2004) A systematic review of molecular and biological tumor markers in neuroblastoma. Clin Cancer Res 10: 4–12
Rubie H, Delattre O, Hartmann O, Combaret V, Michon J, Benard J, Peyroulet MC, Plantaz D, Coze C, Chastagner P, Baranzelli MC, Frappaz D, Lemerle J, Sommelet D (1997a) Loss of chromosome 1p may have a prognostic value in localised neuroblastoma: results of the French NBL 90 Study. Neuroblastoma Study Group of the Societe Francaise d'Oncologie Pediatrique (SFOP). Eur J Cancer 33: 1917–1922
Rubie H, Hartmann O, Michon J, Frappaz D, Coze C, Chastagner P, Baranzelli MC, Plantaz D, Avet-Loiseau H, Benard J, Delattre O, Favrot M, Peyroulet MC, Thyss A, Perel Y, Bergeron C, Courbon-Collet B, Vannier JP, Lemerle J, Sommelet D (1997b) N-Myc gene amplification is a major prognostic factor in localized neuroblastoma: results of the French NBL 90 study. Neuroblastoma Study Group of the Societe Francaise d'Oncologie Pediatrique. J Clin Oncol 15: 1171–1182
Russell HV, Golding LA, Suell MN, Nuchtern JG, Strother DR (2005) The role of bone marrow evaluation in the staging of patients with otherwise localized, low-risk neuroblastoma. Pediatr Blood Cancer 45: 916–919
Sano H, Bonadio J, Gerbing RB, London WB, Matthay KK, Lukens JN, Shimada H (2006) International neuroblastoma pathology classification adds independent prognostic information beyond the prognostic contribution of age. Eur J Cancer 42: 1113–1119
Schleiermacher G, Michon J, Huon I, d'Enghien CD, Klijanienko J, Brisse H, Ribeiro A, Mosseri V, Rubie H, Munzer C, Thomas C, Valteau-Couanet D, Auvrignon A, Plantaz D, Delattre O, Couturier J (2007) Chromosomal CGH identifies patients with a higher risk of relapse in neuroblastoma without MYCN amplification. Br J Cancer 97: 238–246
Simon T, Hero B, Hunneman DH, Berthold F (2003) Tumour markers are poor predictors for relapse or progression in neuroblastoma. Eur J Cancer 39: 1899–1903
Simon T, Spitz R, Faldum A, Hero B, Berthold F (2004) New definition of low-risk neuroblastoma using stage, age, and 1p and MYCN status. J Pediatr Hematol Oncol 26: 791–796
Simon T, Spitz R, Hero B, Berthold F, Faldum A (2006) Risk estimation in localized unresectable single copy MYCN neuroblastoma by the status of chromosomes 1p and 11q. Cancer Lett 237: 215–222
Spitz R, Betts DR, Simon T, Boensch M, Oestreich J, Niggli FK, Ernestus K, Berthold F, Hero B (2006) Favorable outcome of triploid neuroblastomas: a contribution to the special oncogenesis of neuroblastoma. Cancer Genet Cytogenet 167: 51–56
Spitz R, Hero B, Ernestus K, Berthold F (2003) Deletions in chromosome arms 3p and 11q are new prognostic markers in localized and 4s neuroblastoma. Clin Cancer Res 9: 52–58
Swerts K, Ambros PF, Brouzes C, Navarro JM, Gross N, Rampling D, Schumacher-Kuckelkorn R, Sementa AR, Ladenstein R, Beiske K (2005) Standardization of the immunocytochemical detection of neuroblastoma cells in bone marrow. J Histochem Cytochem 53: 1433–1440
Swerts K, De Moerloose B, Dhooge C, Vandesompele J, Hoyoux C, Beiske K, Benoit Y, Laureys G, Philippe J (2006) Potential application of ELAVL4 real-time quantitative reverse transcription-PCR for detection of disseminated neuroblastoma cells. Clin Chem 52: 438–445
Takita J, Ishii M, Tsutsumi S, Tanaka Y, Kato K, Toyoda Y, Hanada R, Yamamoto K, Hayashi Y, Aburatani H (2004) Gene expression profiling and identification of novel prognostic marker genes in neuroblastoma. Genes Chromosomes Cancer 40: 120–132
Trager C, Kogner P, Lindskog M, Ponthan F, Kullman A, Kagedal B (2003) Quantitative analysis of tyrosine hydroxylase mRNA for sensitive detection of neuroblastoma cells in blood and bone marrow. Clin Chem 49: 104–112
Vandesompele J, Baudis M, De Preter K, Van Roy N, Ambros PF, Bown N, Brinkschmidt C, Christiansen H, Combaret V, Lastowska M, Nicholson J, O'Meara A, Plantaz D, Stallings R, Brichard B, Van den Broecke C, De Bie S, De Paepe A, Laureys G, Speleman F (2005) Unequivocal delineation of clinicogenetic subgroups and development of a new model for improved outcome prediction in neuroblastoma. J Clin Oncol 23: 2280–2299
Acknowledgements
This study was supported in part by grants from Fondazione Italiana per la Lotta al Neuroblastoma, Fondazione CA.RI.GE, and Ministry of Health, Italy. SP, FN and BC are recipients of Fondazione Italiana per la Lotta al Neuroblastoma fellowships. The precious INBR data management of Mr Filippo Papio and Ms Barbara Galleni is deeply acknowledged. We thank Veronica Tintori and Cinzia Marchi for providing images of GD2-IC, Bruno De Bernardi for his criticism in reviewing the manuscript and all the physicians who have centralized bone marrow samples for GD2-IC analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Corrias, M., Parodi, S., Haupt, R. et al. Detection of GD2-positive cells in bone marrow samples and survival of patients with localised neuroblastoma. Br J Cancer 98, 263–269 (2008). https://doi.org/10.1038/sj.bjc.6604179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604179
Keywords
This article is cited by
-
Prognostic value of initial bone marrow disease detection by multiparameter flow cytometry in children with neuroblastoma
Journal of Cancer Research and Clinical Oncology (2019)
-
Minimal disease detection in peripheral blood and bone marrow from patients with non-metastatic neuroblastoma
Journal of Cancer Research and Clinical Oncology (2011)