Abstract
In 212 postmenopausal women with node-positive oestrogen receptor-positive (ERLBA) breast cancer subjected to radical surgery and adjuvant tamoxifen, the risk of 6-year relapse increased with increasing values of intratumoral vascular endothelial growth factor (VEGF) in patients whose tumours had a low/intermediate ERLBA content compared to patients with high-ERLBA tumours. These findings indicate that tumour progression, activated or sustained by high VEGF levels, may be counteracted in high-ERLBA cancers by tamoxifen, which in contrast fails to contrast the metastatic potential in low-ERLBA tumours.
Similar content being viewed by others
Main
Breast tissue is highly responsive to changes in ovarian hormone concentrations that induce a cyclic remodelling process involving epithelial, stromal and vascular components during each menstrual cycle (Vogel et al, 1981). In particular, the angiogenic turnover is regulated by oestrogens and progesterone, which modulate the expression of vascular endothelial growth factor (VEGF) in the epithelial cells of the terminal ductal–lobular units (Nakamura et al, 1999).
Although the VEGF gene promoter lacks a perfect palindromic oestrogen-responsive element, an analogue of the consensus element that functions as a classical enhancer for oestrogen receptor (ER) has been identified in the 3′-untranslated region (Hyder et al, 2000b).
Angiogenesis is known to represent a fundamental step in tumour progression (Hanahan and Folkman, 1996) and clinical evidence has shown that node-negative (N−) as well as node-positive (N+) breast cancer patients with high intratumoral VEGF concentrations have a significantly shorter relapse-free survival (RFS) (Gasparini et al, 1997, 1999; Linderholm et al, 2000).
Preclinical studies on breast cancer cell lines demonstrated that, as expected, oestrogens rapidly induce VEGF expression, which is blocked by pure oestrogen antagonists (Ruohola et al, 1999; Hyder et al, 2000a).
In a series of N+ ER-positive (ER+, by ligand-binding assay, LBA) postmenopausal women with resectable breast cancer who received adjuvant tamoxifen, we investigated the comprehensive effect on RFS of VEGF content and steroid receptor profile, evaluated in the same cytosolic fraction.
Patients and methods
Patients
The study included postmenopausal patients with primary resectable invasive breast cancer, histologically classified as N+, who underwent surgery at the Istituto Nazionale Tumori in Milan between March 1991 and December 1995 and received only adjuvant tamoxifen (20 mg day−1) for at least 2 years (median duration time, 4 years) because of their positive ER status (ER tumour concentration higher than 10 fmol mg−1 of protein). From a total of 859 N+, ER+ postmenopausal patients, consecutive with respect to steroid receptor determination at the time of diagnosis, 289 were selected on the basis of treatment, histology (pure or mixed ductal or lobular invasive tumours) and follow-up (i.e. a minimum potential of 6 years from the date of surgery to the date of last updating of patient records).
Of the 289 eligible patients, 212 (73%) were available for VEGF evaluation. Their median age was 64 years (range, 50–85); 88 patients (42%) were treated by mastectomy and 124 (58%) by breast-conserving surgery plus radiotherapy. All of them underwent complete axillary lymph node dissection (median number of examined nodes, 18). Most patients had one to three metastatic axillary lymph nodes (139, 66%). Small (⩽2 cm) and large (>2 cm) tumours were equally represented (93, 48% and 99, 52%, respectively). After surgery, the patients were followed at 6-month intervals during the first 5 years and at 12-month intervals thereafter; disease status was assessed by means of physical examination, chest X-ray, bone scan and abdominal sonography. Treatment failure was defined as the first documented evidence of new disease manifestations in locoregional areas (seven cases), distant sites (40 cases), or in the contralateral breast (five cases). Relapse-free survival was calculated as the time elapsed from diagnosis to the date of first recurrence or to the last clinical examination for patients without documented disease manifestation. Median follow-up for the whole series was 68 months (interquartile range, 52–82 months).
Steroid receptor determination by LBA
Steroid receptor content was determined according to the EORTC recommendations and within national (Piffanelli et al, 1991) and international (EORTC Breast cancer co-operative group, 1980) quality control programmes by a double-labelling assay (Coradini et al, 2000), and expressed as fmol mg−1 of protein. Tumours with an ERLBA concentration higher than 10 fmol mg−1 of protein were defined as ER+.
Vascular endothelial growth factor determination
The predominant VEGF isoform, VEGF65 (henceforward referred to as VEGF), was measured by a quantitative enzyme immunoassay technique (Quantikine, human VEGF; R&D Systems, Minneapolis, MN, USA) as described elsewhere (Coradini et al, 2001). Concentrations were expressed as pg of VEGF protein per mg of total protein.
Statistical analysis
The overall association of VEGF level with patient age, tumour size, number of metastatic lymph nodes, ERLBA and PgRLBA content was evaluated by Spearman's rank correlation coefficient.
The effect of VEGF, ERLBA and PgRLBA content on RFS was investigated by multivariate analysis using a Cox regression model in which also the number of metastatic lymph nodes was included. All variables were considered on a continuous scale after logarithmic transformation. Null values for PgRLBA content were arbitrarily set at 1, taking a sensitivity threshold value of 2 fmol mg−1 of protein. According to a previous finding (Coradini et al, 2001), linear terms for log(ERLBA), log(PgRLBA) and log(VEGF) and the interaction between ERLBA and VEGF were included in the model.
The proportional hazard assumption of the Cox model was evaluated and the effect of model terms was tested as previously reported (Coradini et al, 2001).
The library written by Harrell et al (1996) was applied in some steps of the model building procedure and the SAS macro programme RELIMPCR designed by Heinze and Schemper (2001) was adopted for evaluation of the relative prognostic contribution of the covariates.
Results
In this series of ERLBA-positive tumours from postmenopausal patients, the VEGF content ranged from 7 to 2186 pg mg−1 of protein with 52, 95 and 200 as the 25th, 50th and 75th percentiles, respectively; the PgRLBA concentration ranged from 1 to 2563 fmol mg−1 of protein with 40, 111 and 355 as the 25th, 50th and 75th percentiles, respectively. Vascular endothelial growth factor content did not show any significant correlation with any of the other variables considered; estimated correlation coefficients were all in the range ±0.08.
In the multivariate model, the number of metastatic lymph nodes, VEGF, ERLBA and the interaction between ERLBA and VEGF were significantly related to prognosis (Table 1), whereas no significant contribution was observed for PgRLBA. As for ERLBA, VEGF and the ERLBA–VEGF interaction, the results were similar to those previously obtained in patients with N– breast cancer who did not receive systemic treatment (Coradini et al, 2001). This finding further supports the role of ERLBA in modulating the prognostic effect of VEGF and the negative interaction term indicates a decrease in the unfavourable effect of VEGF for increasing values of ERLBA.
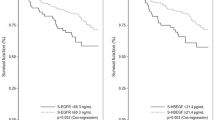
To provide a description of the combined effect of VEGF and ERLBA, the relative hazard (RH) for increasing VEGF concentrations was plotted (Figure 1) for selected values of ERLBA (70 and 220 fmol mg−1 of protein) that were approximately equal to the 1st and third quartiles of ERLBA distribution. Owing to the interaction between ERLBA and VEGF, when the ERLBA content was set at 70 fmol mg−1 protein, the increase in the risk of disease recurrence for high VEGF values associated with low ERLBA values was evident. In fact, the estimated RH for patients with a VEGF content of 200 vs those with a VEGF content of 50 pg mg−1 protein was 1.54 (95% CI, 1.07–2.20). Conversely, when the ERLBA content was set at 220 fmol mg−1 protein, VEGF did not show any prognostic effect and the estimated RH for the two selected values (200 and 50 pg ml−1) was 0.89 (95% CI, 0.54–1.47).
Plots of the logarithm of the relative hazard of disease recurrence as a function of VEGF level for different ERLBA values (fixed approximately at the first and third quartiles of the distribution). The solid line corresponds to an ERLBA=70 fmol mg−1 protein, whereas the dashed line corresponds to an ERLBA=220 fmol mg−1 protein. Dotted lines indicate 95% pointwise confidence limits (upper: UCL; lower: LCL).
Analysis of the prognostic contribution of the covariates showed that the model explained only 13.2% of the total heterogeneity of the relapse times of our case series and that, considering the relative importance of prognostic factors, 8.2% was attributable to the number of metastatic lymph nodes, whereas VEGF and ERLBA together contributed with 3.3%, and PgR only with 0.8%.
Discussion
According to the recently revised treatment guidelines for early breast cancer (Goldhirsch et al, 2001), postmenopausal women with ER+ primary breast cancer should receive adjuvant hormone therapy with tamoxifen. However, a recent clinical study (Linderholm et al, 2000) provided evidence that also the intratumoral VEGF content could predict outcome following adjuvant endocrine treatment: patients with ER+ tumours, but a high VEGF expression, had a significantly shorter RFS and overall survival. In agreement with these findings, our results showed that, due to the presence of a negative interaction between ERLBA and VEGF, already observed in a series of N− cancers (Coradini et al, 2001), patients whose tumours had a low/intermediate ERLBA content exhibited an increased risk of disease recurrence with increasing values of intratumoral VEGF. Conversely, the VEGF level did not show prognostic effect in patients whose tumours had a high ERLBA content. These findings suggest that in tumours characterised by a high ERLBA concentration and therefore more likely to be hormonally regulated, tumour progression, activated or sustained by VEGF, may be counteracted by the protective effect of endocrine therapy. Conversely, when a patient has a high level of VEGF and a low ERLBA concentration, tamoxifen could fail to contrast the tumour's metastatic potential and more tailored adjuvant treatment would be required including, for example, an antiangiogenic agent.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Coradini D, Boracchi P, Daidone MG, Pellizzaro C, Miodini P, Ammatuna M, Tomasic G, Biganzoli E (2001) Contribution of vascular endothelial growth factor to the Nottingham prognostic index in node-negative breast cancer. Br J Cancer 85: 795–797
Coradini D, Daidone MG, Boracchi P, Biganzoli E, Oriana S, Bresciani G, Pellizzaro C, Tomasic G, Di Fronzo G Marubini E (2000) Time-dependent relevance of steroid receptors in breast cancer. J Clin Oncol 18: 2702–2709
EORTC Breast Cancer Co-operative Group (1980) Revision of the standards for the assessment of hormone receptors in human breast cancer. Report of the second EORTC Workshop, held on 16–17 March 1979, in the Netherlands Cancer Institute. Eur J Cancer 16: 1513–1515
Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, Matsubara I, Vinante O, Bonoldi E, Boracchi P, Gatti C, Suzuki H, Tominaga T (1997) Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst 89: 139–147
Gasparini G, Toi M, Miceli R, Vermeulen PB, Dittadi R, Biganzoli E, Morabito A, Fanelli M, Gatti C, Suzuki H, Tominaga T, Dirix LY, Gion M (1999) Clinical relevance of vascular endothelial growth factor and thymidine phosphorylase in patients with node-positive breast cancer treated with either adjuvant chemotherapy or hormone therapy. Cancer J Sci Am 5: 101–119
Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn H-J (2001) Meeting highlights: International Consensus Panel on the treatment of primary breast cancer. J Clin Oncol 19: 3817–3827
Grambsch P, Therneau T (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81: 515–526
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86: 353–364
Harrell Jr FE, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387
Heinze G, Schemper M (2001) RELIMPCR, and RELIMPLR SAS-macros for the analysis of relative importance of prognostic factors in Cox and logistic regression. Vienna: University of Vienna. Department of Medical Computer Sciences, Section of Clinical Biometrics, Technical Report 3/2001
Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, Stancel GM (2000a) Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect 108: 785–790
Hyder SM, Nawaz Z, Chiappetta C, Stancel GM (2000b) Identification of functional response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res 60: 3183–3190
Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Heriksson R (2000) Correlation of vascular endothelial growth factor content with recurrences, survival and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol 18: 1423–1431
Nakamura J, Lu Q, Aberdeen G, Albrecht E, Brodie A (1999) The effect of estrogen on aromatase and vascular endothelial growth factor messenger ribonucleic acid in the normal nonhuman primate mammary gland. J Clin Endocrinol Metab 84: 1432–1437
Piffanelli A, Giovannini G, Pelizzola D, De Bortoli M, Catozzi L, Giganti M (1991) Steroid receptor assays: an Italian quality assessment program. Ann Ist Super Sanità 27: 523–529
Ruohola JK, Valve EM, Karkkainen MJ, Joukov V, Alitalo K, Harkonen PL (1999) Vascular endothelial growth factors are differentially regulated by steroid hormones and antiestrogens in breast cancer cells. Mol Cell Endocrinol 25: 29–40
Vogel PM, Georgiade NG, Fetter BF, Vogel FS, Mc Carty Jr KS (1981) The correlation of histologic changes in human breast with the menstrual cycle. Am J Pathol 104: 23–34
Acknowledgements
This study was supported by a grant from the Consiglio Nazionale delle Ricerche (CNR) and the Italian Association for Cancer Research (AIRC), Italy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Coradini, D., Biganzoli, E., Pellizzaro, C. et al. Vascular endothelial growth factor in node-positive breast cancer patients treated with adjuvant tamoxifen. Br J Cancer 89, 268–270 (2003). https://doi.org/10.1038/sj.bjc.6601060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601060
Keywords
This article is cited by
-
Angiogenesis-associated sequence variants relative to breast cancer recurrence and survival
Cancer Causes & Control (2010)
-
Shorter survival-times following adjuvant endocrine therapy in oestrogen- and progesterone-receptor positive breast cancer overexpressing HER2 and/or with an increased expression of vascular endothelial growth factor
Medical Oncology (2009)
-
Tumor-specific VEGF-A and VEGFR2 in postmenopausal breast cancer patients with long-term follow-up. Implication of a link between VEGF pathway and tamoxifen response
Breast Cancer Research and Treatment (2005)