Abstract
Backgroud:
Constitutive activation of TGF-β signalling is a well-recognised mechanism in bone metastasis of prostate cancer (PCa). Protein Interacting with PRKCA 1 (PICK1) is a critical negative regulator of the TGF-β pathway. However, the clinical significance and biological role of PICK1 in PCa bone metastasis remain obscure.
Methods:
PICK1 expression is evaluated by immunohistochemistry (IHC) in 198 PCa patients. Statistical analysis is performed to explore correlation between PICK1 expression and clinicopathological features in PCa patients. The biological role of PICK1 is examined in PC-3 and C4-2B cells in vitro and a mouse intracardial model in vivo.
Results:
PICK1 expression is decreased in PCa tissues with bone metastasis and bone-derived cells and downregulation of PICK1 positively correlates with serum PSA level, Gleason grade and bone metastasis status in PCa patients. Overexpression of PICK1 suppresses PCa cell invasion and migration in vitro and bone metastasis in vivo. Our results further indicate downregulation of PICK1 is caused by miR-210-3p overexpression in PCa tissues with bone metastasis. Clinical negative correlation of PICK1 with miR-210-3p is confirmed in PCa tissues.
Conclusions:
Our findings uncover a novel functionally and clinically relevant epigenetic regulatory mechanism for constitutive activation of TGF-β signalling in bone metastasis of PCa.
Similar content being viewed by others
Main
Prostate cancer (PCa) is one of the most commonly diagnosed cancers with indolent malignant features in men (Nelson et al, 2003). Despite great progress in the systemic treatment of PCa in recent years, distant bone metastasis remains the primary issue affecting the quality of life and survival time of PCa patients (Bubendorf et al, 2000). Therefore, a better understanding of the underlying mechanisms contributing to bone metastasis of PCa will facilitate the development of novel therapeutic strategies against PCa.
The TGF-β signalling pathway has important functions in several biological processes, including inhibiting cell proliferation, inducing cell differentiation, embryogenesis and bone remodelling (Mohammad et al, 2009). In cancer, TGF-β signalling can promote or inhibit tumourigenesis depending on the developmental stage and type of tumour (Siegel and Massague, 2003; Jakowlew, 2006). Importantly, it has been found that TGF-β signalling plays crucial roles in cancer metastasis, especially to bone (Kang et al, 2005). Many studies to date have reported that the development of osteolytic bone disease during bone metastasis accompanied by the release of various factors, especially TGF-β, from stromal cells and the bone microenvironment enhances tumour cell proliferation and survival, leading to a vicious cycle (Ell and Kang, 2012). Except for the well-documented roles of TGF-β signalling within the bone microenvironment, considerable attention has been given to the pro-bone metastasis roles of TGF-β signalling, where activation of TGF-β signalling drives tumour cell invasion and migration (Wang et al, 2015; Yu et al, 2016). For example, Fournier and colleagues reported that activation of TGF-β signalling upregulated PMEPA1, an important negative regulator of the TGF-β pathway, and that interrupting this negative feedback loop by PMEPA1 knockdown caused PCa cells to disseminate to bone marrow, ultimately increasing bone metastases in a mouse PCa model (Fournier et al, 2015). Furthermore, it is reported that therapy targeting TGF-β significantly attenuates metastasis of tumour cells to bone (Juarez and Guise, 2011; Hu et al, 2012; Wan et al, 2012). Although these studies demonstrate that the TGF-β pathway plays crucial roles in the bone metastasis of PCa, the molecular mechanism responsible for constitutive activation of TGF-β in PCa bone metastasis needs to be further elucidated.
Protein Interacting with PRKCA 1 (PICK1), which was initially found by a yeast two-hybrid system analysis for identifying proteins that interact with activated protein kinase C, alpha (PRKCA) (Staudinger et al, 1995), functions as an adaptor that binds to and guides the subcellular localisation and distribution of a set of membrane proteins (Staudinger et al, 1995). Previous studies have shown that PICK1 has important roles in regulating the cytoskeleton and neuron morphology, and PICK1 has been implicated in several diseases (Steinberg et al, 2006; Nakamura et al, 2011; Rocca et al, 2013; Rocca and Hanley, 2015; Kunicka et al, 2016). Recent studies implicate PICK1 dysregulation in the progression and metastasis of cancers (Cockbill et al, 2015). Notably, a study from Zhao et al reported that PICK1 acts as an important negative regulator of TGF-β signalling in breast cancer cells by serving as a scaffold protein to enhance interaction between TGF-β receptor I (TβRI) and caveolin-1. Such interaction leads to enhanced lipid raft/caveolae localisation, resulting in caveolin-mediated endocytosis, ubiquitination and degradation of TβRI (Zhao et al, 2012). However, the clinical significance and biological roles of PICK1 in bone metastasis of PCa are largely unknown.
MicroRNAs, which exhibit potent repressive activity on target genes by binding to the 3' untranslated region (3'UTR) of messenger RNA (mRNA), are reported to be important mediators in bone metastasis of PCa (Wang et al, 2008; Pang et al, 2010; Guo et al, 2013; Ren et al, 2013, 2014). In this study, we found that PICK1 expression is decreased in PCa tissues with bone metastasis and bone metastatic cells and that reduced PICK1 expression positively correlates with the clinicopathological characteristics and bone metastasis status of PCa patients. Upregulation of PICK1 suppresses invasion and migration in vitro, as well as bone metastasis in PCa cells in vivo through repression of TGF-β signalling. Furthermore, our results demonstrate that high miR-210-3p expression is the main underlying epigenetic regulatory mechanism responsible for downregulation of PICK1 in PCa tissues with bone metastasis. Thus, our findings reveal a novel mechanism of constitutive activation of TGF-β signalling, resulting in the development of bone metastasis of PCa.
Materials and methods
Cell lines and cell culture
The human PCa cell lines 22RV1, PC-3, VCaP, DU145, and LNCaP and normal prostate epithelial cells RWPE-1 were obtained from the Shanghai Chinese Academy of Sciences cell bank (China). RWPE-1 cells were grown in Defined Keratinocyte-SFM (1 ×) (Invitrogen, USA). PC-3, LNCaP and 22Rv1 cells were cultured in RPMI-1640 medium (Life Technologies, USA) supplemented with penicillin G (100 U ml–1), streptomycin (100 mg ml–1) and 10% fetal bovine serum (FBS, Life Technologies, USA). DU145 and VCaP cells were grown in Dulbecco's modified Eagle’s medium (Invitrogen, USA) supplemented with 10% FBS. The C4-2B cell line was purchased from MD Anderson Cancer Center and maintained in T-medium (Invitrogen, USA) supplemented with 10% FBS (Wu et al, 1998). All cell lines were authenticated by short-tandem repeat (STR) fingerprinting at Medicine Lab of Forensic Medicine Department of Sun Yat-Sen University (China). All cell lines were grown under a humidified atmosphere of 5% CO2 at 37 °C.
Plasmid, small interfering RNA and transfection
Human PICK1 cDNA (Vigene Biosciences, China) was cloned into the pSin-EF2 vector (Cambridge, USA). The (CAGAC) 12/pGL3 TGF-β/Smad-responsive luciferase reporter plasmid and control plasmids (Clontech, Japan) were used to quantitatively assess the transcriptional activity of TGF-β signalling components. The 3′UTR region of PICK1 was polymerase chain reaction (PCR) amplified from genomic DNA and cloned into pmirGLO vectors (Promega, USA). The list of primers used is presented in Supplementary Table S2. Antagomir-210-3p, small interfering RNA (siRNA) targeting PICK1 and respective control RNA were synthesised and purified by RiboBio. Transfection of miRNA, siRNA, and plasmids was performed using Lipofectamine 3000 (Life Technologies, USA) according to the manufacturer’s instructions.
RNA extraction, reverse transcription, and real-time PCR
Total RNA from tissues or cells was extracted using RNA Isolation Kit (Qiagen, USA) according to the manufacturer’s instructions. mRNA and miRNA were reverse transcribed from total mRNA using RevertAid First Strand cDNA Synthesis Kit (Thermo, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was amplified and quantified using a CFX96 system (BIO-RAD, USA) with iQ SYBR Green (BIO-RAD, USA). The primers are provided in Supplementary Table S1. Primers for U6 and miR-210-3p were synthesised and purified by RiboBio (Guangzhou, China). The expression of miRNA was defined based on Ct, and relative expression levels were calculated as 2−(Ct miR-210-3p −Ct U6) after normalisation with reference to the quantification of U6 small nuclear RNA expression and expression levels of mRNA were calculated after normalisation with reference to the quantification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA expression according to the previous study (Song et al, 2012).
Western blotting
Nuclear/cytoplasmic fractionation was performed using Cell Fractionation Kit (Cell Signaling Technology, USA) according to the manufacturer’s instructions, and whole-cell lysates were extracted using RIPA Buffer (Cell Signaling Technology, USA). Western blotting was performed according to a standard method, as described previously (Wang et al, 2016). Antibodies against PICK1, pSMAD2/3, SMAD2/3 and p84 were purchased from Cell Signaling Technology (USA). As a loading control, membranes were stripped and reprobed with an anti-α-tubulin antibody (Sigma-Aldrich, USA).
Luciferase assay
Cells (4 × 104) were seeded in triplicate in 24-well plates, cultured for 24 h, and transfected with 250 ng (CAGAC) 12/pGL3 reporter luciferase plasmid or 100 ng pmirGLO-PICK1-3′UTR, luciferase plasmid, and 5 ng pRL-TK Renilla plasmid (Promega, USA) using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s recommendations. The luciferase and Renilla signals were measured 36 h after transfection using a Dual Luciferase Reporter Assay Kit (Promega, USA) according to the manufacturer’s protocol.
miRNA immunoprecipitation
The MS2bp-MS2bs-based miRNA immunoprecipitation (RIP) assay was performed according to previous reports (Gong and Maquat, 2011), with modifications for the use of EZ-MagnaRIP Kit (Millipore, USA) in accordance with the manufacturer’s instructions.
Migration and invasion assays
Invasion and migration assays were performed using a Transwell chamber consisting of 8-mm membrane filter inserts (Corning, USA) with or without Matrigel (BD Biosciences, USA) coating. Briefly, cells were trypsinised and suspended in serum-free medium, and a quantity of 1.5 × 105 cells was added to the upper chamber; the lower chamber was fill+ed with the culture medium plus 10% FBS. After incubation for 48 h, the cells that had passed through the coated membrane to the lower surface were fixed with 4% paraformaldehyde and stained with hematoxylin. The cells were counted under a microscope (× 100).
Animal study
All mouse experiments were approved by The Institutional Animal Care and Use Committee of Sun Yat-sen University; the Approval no. was L102012013004A. For a bone metastasis study, BALB/c-nu mice (5–6 weeks old, 18–20 g) were anesthetised and inoculated into the left cardiac ventricle with 1 × 105 PC-3 cells in 100 μl of phosphate-buffered saline (PBS). Bone metastases were monitored by bioluminescence imaging (BLI), as previously described (Liu et al, 2013). The development of bone lesions was evaluated by radiography using a Faxitron MX-20 with a digital camera (Faxitron Bioptics, USA). Osteolytic lesions were identified on radiographs as radiolucent lesions in the bone. The area of the osteolytic lesions was measured using the image analysis system MetamorphAnalysis Software (Universal Imaging Corporation, USA), and the total extent of bone destruction per animal is expressed in square millimeters. Each bone metastasis was scored as follows: 0, no metastasis; 1, bone lesion covering <1/4 of the bone width; 2, bone lesion involving 1/4–1/2 of the bone width; 3, bone lesion across 1/2–3/4 of the bone width; and 4, bone lesion >3/4 of the bone width. The bone metastasis score for each mouse was the sum of scores of all bone lesions from four limbs.
Patients and tumour tissues
A total of 198 paraffin-embedded, archived PCa tissues, 10 metastatic bone tissues and 51 fresh PCa tissues were obtained during surgery or needle biopsy between January 2014 and October 2016. Patients were diagnosed based on clinical and pathological evidence, and the specimens were immediately snap-frozen and stored in liquid nitrogen tanks. Before patient consent and approval from the Institutional Research Ethics Committee were obtained for the use of these clinical materials for research purposes. The clinicopathological features of the patients are summarised in Supplementary Table S3.
Immunohistochemistry
The immunohistochemistry procedure and scoring of PICK1 expression levels were performed as previously described (Zhang et al, 2017). Scores given by two independent investigators were averaged for further comparative evaluation of PICK1 expression. The proportion of tumour cells was scored as follows: 0 (no positive tumour cells); 1 (<10% positive tumour cells); 2 (10–35% positive tumour cells); 3 (35–70% positive tumour cells) and 4 (>70% positive tumour cells). The staining intensity was graded according to the following criteria: 0 (no staining); 1 (weak staining, light yellow); 2 (moderate staining, yellow brown) and 3 (strong staining, brown). The staining index (SI) was calculated as the product of the staining intensity score and the proportion of positive tumour cells. Using this method of assessment, we evaluated PICK1 expression in PCa samples by determining SI, with scores of 0, 1, 2, 3, 4, 6, 8, 9 or 12. An SI score 4 was the median SI of all sample tissues. High and low PICK1 expression was stratified by the follow criteria: SI⩽4 was used to define tumours with low PICK1 expression; SI >6 was used to define tumours with high-PICK1 expression.
Statistical analysis
All values are presented as means±s.d. Significant differences were evaluated using GraphPad 5.0 software (USA). Student’s t-test was used to determine significant differences between two groups. The chi-square test was used to analyze the relationship between PICK1 or miR-210-3p expression and clinicopathological characteristics in PCa patients. A two-tailed P-value of <0.05 was considered statistically significant in all experiments. All experiments were repeated three times.
Results
PICK1 is downregulated in PCa tissues with bone metastasis and bone metastatic cell lines
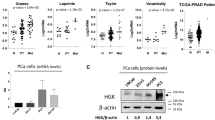
To determine the clinical significance of PICK1 in PCa, we first analysed a publicly available PCa mRNA sequencing dataset and found that PICK1 expression was dramatically decreased in PCa tissues with bone metastasis compared with PCa tissues without bone metastasis (Figure 1A and B). We further examined expression of PICK1 in our 51 fresh PCa tissues and found it to be significantly lower in PCa tissues with bone metastasis compared with PCa tissues without bone metastasis (Figure 1C). In addition, the percentage of low-PICK1 expression was higher in PCa tissues with bone metastasis compared to PCa tissues without bone metastasis (Supplementary Figure 1A and B). Western blot analysis revealed downregulation of PICK1 protein expression in PCa tissues with bone metastasis (Figure 1D). Consistently, the mRNA and protein levels of PICK1 in PCa cell lines derived from bone metastases (PC-3, C4-2B and VCaP) were significantly lower than those in 22RV1 cells derived from primary PCa, DU145 cells derived from brain metastasis and LNCaP cells derived from lymph node metastasis (Figure 1E and Supplementary Figure 1C). Immunohistochemical (IHC) staining of PICK1 in 198 PCa tissues was performed. The results showed that PICK1 was strongly expressed in PCa tissues without bone metastasis, but notably downregulated in PCa tissues with bone metastasis and further decreased in PCa bone tissues (Figure 1F). Through further analysis of the relationship between PICK1 expression and clinicopathological characteristics, we found that the level of PICK1 negatively correlated with serum prostate-specific antigen (PSA) levels (P<0.001), the Gleason grade (P=0.015) and the bone metastasis status (P<0.001) in PCa (Supplementary Tables S3 and S4). Collectively, these results implicate reduced PICK1 expression in the bone metastasis of PCa.
PICK1 is downregulated in PCa tissues with bone metastasis. (A) PICK1 expression was dramatically reduced in PCa tissues with bone metastasis (PCa/BM) compared with PCa tissues without bone metastasis (PCa/nBM) based on analysis of the TCGA PCa sequencing dataset (PCa/nBM, n=12; PCa/BM, n=10). P=0.002. (B) PICK1 expression was dramatically decreased in PCa/BM compared with PCa/nBM based on analysis of the GSE46602 PCa sequencing dataset (PCa/nBM, n=14; PCa/BM, n=22). P=0.003. (C) Real-time PCR analysis of PICK1 expression in 23 freshly collected non-bone metastatic and 28 bone metastatic PCa tissue samples. Transcript levels were normalised to GAPDH expression. Lines represent median and lower/upper quartiles. P<0.001. (D) Western blotting analysis of PICK1 expression in 4 PCa/nBM and 4 PCa/nBM. α-Tubulin served as the loading control. (E) Western blotting analysis of PICK1 expression in PCa cells. α-Tubulin served as the loading control. (F) Immunohistochemical staining of PICK1 protein expression in representative samples of PCa/nBM, PCa/BM and bone of PCa are shown.
PICK1 inhibits bone metastasis of PC-3 cells
To determine the role of PICK1 in PCa bone metastasis in vivo, we first generated PCa cell lines stably overexpressing PICK1 by ectopically overexpressing PICK1 via retrovirus infection (Supplementary Figure 2). A mouse model of bone metastasis was employed, whereby a luciferase control or PICK1-overexpressing PC-3 cells were inoculated into the left cardiac ventricle of male nude mice to monitor the development of distant bone metastasis loci by BLI. As shown in Figure 2A and B, PICK1-overexpressing PC-3 cells caused fewer bone metastases compared with the control group by both X-ray and BLI. Hematoxylin and eosin (H&E) staining of sectioned tumours from the tibias of injected mice demonstrated that increased PICK1 expression significantly decreased the tumour burden in bone (Figure 2C). Furthermore, PICK1-overexpressing cells resulted in fewer metastatic foci and smaller osteolytic areas of metastatic tumours, as well as longer survival and bone metastasis-free survival rates compared to the control group (Figure 2D–G). Therefore, these findings indicate that upregulation of PICK1 inhibits the bone metastasis of PCa.
Overexpression of PICK1 inhibits bone metastasis of PC-3 cells in vivo. (A) Representative BLI signal of bone metastasis in a mouse from the indicated groups of mice at 3 min and 8 weeks. (B) Representative radiographic images of bone metastases in the indicated mice (arrows indicate osteolytic lesions). (C) Representative H&E-stained sections of tibias from the indicated mouse (T, tumour; N, the adjacent non-tumour tissues). (D) The sum of bone metastasis scores for each mouse among tumour-bearing mice inoculated cells carrying the vector (n=8) or a PICK1-overexpression plasmid (n=9). *P<0.05. (E) Quantification of BLI signals in the vector and PICK1-overexpression groups at 6, 7 and 8 weeks. *P<0.05. (F) Kaplan–Meyer analysis of mouse survival for the vector and PICK1-overexpression groups. (G) Kaplan–Meyer analysis of mouse bone metastasis-free survival for the vector and PICK1-overexpression groups.
PICK1 inhibits TGF-β signalling activity in PCa cells
Emerging evidence demonstrates that PICK1 is a critical negative regulator of the TGF-β pathway (Zhao et al, 2012); thus, we examined the effect of PICK1 on TGF-β signalling. As shown in Figure 3A, upregulating PICK1 suppressed the transcriptional activity of the TGF-β/Smad-responsive luciferase reporter plasmid CAGA12, which consists of 12 tandem copies of the Smad/DNA binding motif CAGAC. Cellular fractionation and western blotting analysis revealed that upregulation of PICK1 decreased pSMAD2/3 nuclear translocation in PCa cells (Figure 3B). Moreover, real-time PCR analysis showed that PICK1 overexpression repressed multiple downstream bone metastasis-related genes of the TGF-β pathway in both the absence and presence of ectopic TGF-β (Figure 3C–K). Overall, our results demonstrate that PICK1 inhibits TGF-β signalling activity in PCa cells.
Overexpression of PICK1 represses TGF- β signalling activity in PCa cells. (A) Upregulation of PICK1 transcriptional activity based on a TGF-β/Smad-responsive luciferase reporter in the absence or presence of TGF-β (5 ng ml–1). *P<0.05, **P<0.01. (B) Western blot analysis showing that upregulation of PICK1 decreased nuclear translocation of pSMAD2/3 in PCa cells in the absence or presence of TGF-β. The nuclear protein p84 was used as a nuclear protein marker. (C–K) Overexpressing PICK1 repressed downstream bone metastasis-related genes of the TGF-β pathway, including CTGF, PTHRP, IL-11, NEDD9, MMP13, ADAM19, THBS1, COL1A1 and VEGFA, in the absence or presence of TGF-β. Transcript levels were normalised to GAPDH expression. Error bars represent the mean±s.d. of three independent experiments. *P<0.05, **P<0.01.
Furthermore, in vitro invasion and migration assays showed that upregulating PICK1 inhibited the invasion and migration capacities of PCa cells both in the absence and presence of TGF-β (Supplementary Figure 3A and B). These results indicate that PICK1 downregulation promotes invasion and migration in PCa cells by activating TGF-β signalling.
Overexpression of miR-210-3 causes PICK1 downregulation in PCa tissues with bone metastasis
To clarify the underlying mechanism of reduced PICK1 expression in PCa tissues with bone metastasis, we first analyzed the deletion status from a genetic perspective in a PCa dataset from The Cancer Genome Atlas (TCGA) and found a deletion rate of only 0.2% among all 496 TCGA PCa samples (Supplementary Figure 4A). This result indicates that epigenetic regulation may be involved in PICK1 downregulation in PCa tissues with bone metastasis. We further analysed methylation levels of PICK1 in PCa tissues. According to our results, there was no found obvious difference between adjacent non-tumourous tissues (ANT) and PCa tissues, PCa tissues with bone metastasis and PCa tissues without bone metastasis regarding PICK1 methylation levels (Supplementary Figure 4B and C). This finding suggests that other mechanisms are responsible for the reduced expression of PICK1 in PCa tissues with bone metastasis.
As miRNAs, small endogenous non-coding RNAs that post transcriptionally regulate target genes by binding to their 3′UTR, leading to degradation, are involved in cancer (Bartel, 2009), we investigated whether miRNAs participate in PICK1 downregulation in PCa tissues with bone metastasis. Using the publicly available algorithms TargetScan and miRanda, we identified 29 broadly conserved miRNAs with the potential to bind to the 3′UTR of PICK1. To validate the ability of these predicted miRNAs to directly bind to the PICK1 3′UTR, an RNA immunoprecipitation (RIP) analysis with the MS2 binding protein (MS2bp) was performed. A plasmid expressing the 3′UTR of PICK1 containing the MS2-binding sequence was constructed and cotransfected together with the MS2bp-YFP expression vector into PC-3, C4-2B and VCaP cells. The transcript-specific binding of YFP protein in combination with miRNA complexes was precipitated with a YFP antibody. Quantitative real-time PCR analysis revealed strong enrichment of nine miRNAs in binding complexes, indicating the potent binding ability of these miRNAs to the PICK1 3′UTR (Figure 4A). We further evaluated these nine miRNAs and found that only miR-338-3p, miR-210-3p and miR-34a-5p significantly correlated with bone metastasis of PCa in the TCGA PCa dataset (Figure 4B). As miRNAs suppress target genes and PICK1 negatively regulates PCa bone metastasis, only those miRNAs that correlated positively with PCa bone metastasis would inhibit PICK1 expression, resulting in bone metastasis of PCa. Accordingly, miR-210-3p was the only potential candidate in this scenario (Figure 4B), and miR-210-3p expression should be elevated in PCa tissues with bone metastasis compared with PCa tissues without bone metastasis. Indeed, publicly available PCa data sets and our PCa tissues revealed significantly elevated levels of miR-210-3p expression in PCa tissues with bone metastasis and bone metastasis cell lines (Figure 4C and D and Supplementary Figure 5). Furthermore, real-time PCR and western blot analysis revealed that enhanced miR-210-3p expression decreased while silenced miR-210-3p increased the mRNA and protein levels of PICK1 in PCa cells (Figure 4E and F). In addition, a luciferase assay using in PCa cells showed that miR-210-3p overexpression attenuated and miR-210-3p inhibition increased reporter activity driven by the PICK1 3′UTR but not a mutant miR-210-3p seed region (Figure 4G). Collectively, these results demonstrate that miR-210-3p overexpression leads to reduced expression of PICK1 in PCa tissues with bone metastasis.
Overexpression of miR-210-3p elicits PICK1 downregulation in PCa tissues with bone metastasis. (A) The binding ability of 29 broadly conserved miRNAs to the PICK13′UTR, as precipitated using cDNA combined with MS2- binding sequences (MS2bs) and the binding protein MS2BP-YFP. The immunoprecipitated miRNAs were assayed by quantitative real-time PCR and normalised to U6. (B) Correlation of miRNAs with the potential ability to bind to the 3′UTR of PICK1 with PCa bone metastasis. *P<0.05. (C) Real-time PCR analysis of miR-210-3p expression in 108 PCa/nBM tissues and 90 PCa/BM tissues. Transcript levels were normalised to U6 expression. Lines represent median and lower/upper quartiles. P<0.001. (D) miR-210-3p expression levels were markedly elevated in PCa/BM tissues compared with PCa/nBM tissues, as assessed by analysis of the TCGA PCa miRNA sequencing dataset (nBM, n=11; BM, n=9). P=0.04. (E) Real-time PCR analysis of PICK1 expression in the indicated cells. Transcript levels were normalised to U6 expression. Error bars represent the mean±s.d. of three independent experiments. *P<0.05. (F) Western blotting of PICK1 expression in the indicated cells. α-Tubulin served as the loading control. (G) Luciferase assay of cells transfected with the pmirGLO-PICK13'UTR reporter in miR-210-3p-overexpressing and -silenced PC-3 and C4-2B cells. *P<0.05.
miR-210-3p activates TGF-β signalling and promotes invasion and migration by targeting PICK1 in PCa cells
We first examined the effects of miR-210-3p on TGF-β signalling activity and migration and invasion abilities in PCa cells. As shown in Figure 5A–E, silencing miR-210-3p inhibited pSMAD2/3 nuclear translocation, TGF-β signalling activity and downstream target gene expression. Moreover, downregulation of miR-210-3p repressed migration and invasion capacities of PC-3 cells (Figure 5F and G). Further analysis showed that silencing PICK1 reversed the inhibitory effects of reduced miR-210-3p expression on pSMAD2/3 nuclear translocation, TGF-β signalling activity and downstream target gene expression in PCa cells. In addition, downregulating PICK1 restored the miR-210-3p silencing-induced inhibition of migration and invasion in PCa cells. These findings demonstrate that miR-210-3p promotes TGF-β signalling in PCa cells, as well as migration and invasion by repressing PICK1.
Silencing PICK1 abrogates the inhibitory effects of miR-210-3p silencing on TGF- β signalling activity, invasion and migration. (A–E) The inhibitory effects of silencing miR-210-3p on pSMAD2/3 nuclear translocation, the transcriptional activity of a TGF-β/Smad-responsive luciferase reporter and bone metastasis-related gene expression in PCa cells were attenuated by PICK1 downregulation. Error bars represent the mean±s.d. of three independent experiments. *P<0.05, **P<0.01. (F and G) The inhibitory effects of silencing miR-210-3p on the invasion and migration abilities of PCa cells were attenuated by PICK1 downregulation. Error bars represent the mean±s.d. of three independent experiments. *P<0.05, **P<0.01.
Clinical correlation of miR-210-3p with PICK1 in human PCa tissues
To investigate the clinical significance of miR-210-3p and PICK1, miR-210-3p expression and the mRNA levels of PICK1 were examined in 51 fresh PCa tissues. As shown in Figure 6A, the negative correlation between PICK1 mRNA and miR-210-3p expression was verified in our PCa tissue samples, consistent with the TCGA PCa tissue analysis (Figure 6B). Indeed, PICK1 expression was inversely correlated with miR-210-3p expression (r=−0.697, P<0.05) and downstream bone metastasis-related genes of TGF-β signalling, CTGF (r=−0.791, P<0.05), PTHRP (r=−0.763, P<0.05) and IL-11 (r=−0.686, P<0.05), in human PCa and bone tissues (Figure 6C–G). Thus, our results indicate that decreased PICK1 expression due to miR-210-3p promotes bone metastasis via TGF-β signalling in PCa (Figure 6H).
Clinical relevance between miR-210-3p and PICK1 in PCa and bone tissues. (A) The clinical negative correlation between miR-210-3p expression and PICK1 mRNA levels was verified in 51 freshly frozen human PCa tissues. Transcript levels were normalised to U6 for miR-210-3p expression and GAPDH for mRNA PICK1 expression. P<0.001. (B) The clinical negative correlation of miR-210-3p expression with PICK1 mRNA levels was analyzed in TCGA PCa tissues. (C) Real-time PCR and western blot analysis of miR-210-3p and PICK1 expression in 3 PCa/nBM tissues, 3 PCa/BM tissues and 3 bone of PCa tissues. Transcript levels were normalised to U6 expression. α-Tubulin was used as the loading control. (D–G) Correlation between PICK1 expression levels and miR-210-3p and downstream bone metastasis-related genes, including CTGF, PTHRP and IL-11, in PCa tissues. Expression levels of PICK1 were quantified by densitometry using Quantity One Software and normalised to the levels of α-tubulin. Sample 1 was used as a standard. The relative expression levels of the proteins were used to perform correlation analysis. (H) Schematic model. Downregulation of PICK1 caused by miR-210-3p activates TGF-β signalling, leading to bone metastasis of PCa.
Discussion
Within the context of cancer progression, emerging evidence implicates PICK1 in the formation of cell–cell boundaries, cell movement, and positioning during development, whereas loss of PICK1 leads to dissociation of epithelial cells via disruption of adherens junctions, resulting in invasion and metastasis of cancer cells (Son et al, 2014). Accordingly, aberrant expression of PICK1 has been linked to more aggressive and metastatic tumour phenotypes. For example, in astrocytic tumours, exogenous expression of PICK1 effectively represses the migration and invasion capacity of cells (Cockbill et al, 2015). However, the clinical significance and biological role of PICK1 in bone metastasis of PCa remain unknown. In this study, we found PICK1 expression to be dramatically decreased in PCa tissues with bone metastasis and in bone metastatic cells and even further decreased in bone tissues of PCa. Furthermore, upregulating PICK1 inhibited invasion and migration abilities in vitro, as well as bone metastasis of PCa cells in vivo. In clinical PCa cases, the level of PICK1 in PCa tissues negatively correlated with the serum PSA level, Gleason grade and bone metastasis status. Thus, our results demonstrate the tumour-suppressive role of PICK1 in the bone metastasis of PCa. Intriguingly, Zhang et al reported overexpression of PICK1 in breast cancer cells, correlating with shortened overall survival. In addition, silencing PICK1 in MDA-MB-231 cells decreased proliferation and tumourigenicity both in vitro and in vivo, supporting an oncogenic role of PICK1 in human breast cancer (Zhang et al, 2010). However, the specific mechanism responsible has not yet been elucidated. Collectively, our results, in combination with other studies, indicate that the pro- and anti-tumour roles of PICK1 are environmental and cancer-type dependent.
Several lines of evidence demonstrate that TGF-β signalling is constitutively activated in bone metastases of various types of cancer, including breast cancer, melanoma and PCa (Yin et al, 1999; Kang et al, 2005; Javelaud et al, 2007). Activation of TGF-β signalling promotes bone metastasis of cancer cells via transcriptional regulation of the expression of multiple bone metastasis-related genes, including CXCR4, MMP1, IL11, and PTHRP (Yin et al, 1999; Kang et al, 2003, 2005). Much research has been conducted regarding the activation of TGF-β signalling in tumour metastasis (Reichl et al, 2015; Tang et al, 2015), and emerging evidence indicates the contribution of several regulatory mechanisms.
Loss or downregulation of upstream negative regulators of the TGF-β pathway is involved in its activation, further promoting the progression and metastasis of tumours. Wang and colleagues reported that EWI-2 negatively regulates TGF-β signalling, which further inhibits the epithelial-mesenchymal transition (EMT), migration and invasion, and lung metastasis of melanoma cells (Wang et al, 2015). In gallbladder carcinoma, reduced SMAD7 expression activates TGF-β signalling, inducing EMT and enhancing metastasis (Chang et al, 2013). Notably, Fournier and colleagues reported that upregulation of the negative regulator of the TGF-β pathway PMEPA1 induced by activation of TGF-β signalling significantly repressed bone metastasis of PCa. In addition, disrupting the negative feedback loop by silencing of PMEPA1 enhanced bone metastases in a mouse prostate cancer model (Fournier et al, 2015). In the present study, our results showed that upregulating PICK1, a negative regulator of the TGF-β pathway, suppressed TGF-β signalling activity, which further inhibited bone metastasis of PCa. These findings indicate that reduced expression of PICK1 in PCa tissues can activate TGF-β signalling and promote PCa bone metastasis.
Recent studies have demonstrated that miRNA dysregulation is another important mechanism responsible for activating TGF-β signalling, which results in tumour cell metastasis in different types of cancer. In gallbladder carcinoma, ectopic expression of miR-20a activated TGF-β signalling by targeting SMAD7, which induced EMT and enhanced metastasis (Chang et al, 2013). Bonci et al reported that concomitant loss of miR-15/miR-16 and gain of miR-21 in PCa cells aberrantly activated TGF-β signalling, which mediated local invasion, distant bone marrow colonisation and osteolysis (Bonci et al, 2016). In this study, we found that overexpression of miR-210-3p activated TGF-β signalling and promoted the bone metastatic capacities of PCa cells in vitro and that silencing of PICK1 activates TGF-β signalling and promotes bone metastatic abilities of PCa cells. Importantly, we found that overexpression of miR-210-3p is the primary mechanism contributing to PICK1 downregulation in bone metastatic PCa tissues. Taken together, our findings reveal a novel mechanism responsible for activating TGF-β signalling, which contributes to bone metastasis of PCa (Figure 6H).
Conclusion
Our results demonstrate that downregulation of PICK1 elicited by miR-210-3p overexpression activates the TGF-β pathway, which further promotes bone metastasis in PCa, indicating that the miR-210-3p/PICK1/TGF-β signalling axis plays an important role in PCa bone metastasis. These findings contribute to a comprehensive understanding of the mechanism responsible for activation of the TGF-β pathway, facilitating the development of an effective therapeutic strategy against bone metastasis of PCa.
References
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136 (2): 215–233.
Bonci D, Coppola V, Patrizii M, Addario A, Cannistraci A, Francescangeli F, Pecci R, Muto G, Collura D, Bedini R, Zeuner A, Valtieri M, Sentinelli S, Benassi MS, Gallucci M, Carlini P, Piccolo S, De Maria R (2016) A microRNA code for prostate cancer metastasis. Oncogene 35 (9): 1180–1192.
Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ (2000) Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum Pathol 31 (5): 578–583.
Chang Y, Liu C, Yang J, Liu G, Feng F, Tang J, Hu L, Li L, Jiang F, Chen C, Wang R, Yang Y, Jiang X, Wu M, Chen L, Wang H (2013) MiR-20a triggers metastasis of gallbladder carcinoma. J Hepatol 59 (3): 518–527.
Cockbill LM, Murk K, Love S, Hanley JG (2015) Protein interacting with C kinase 1 suppresses invasion and anchorage-independent growth of astrocytic tumor cells. Mol Biol Cell 26 (25): 4552–4561.
Ell B, Kang Y (2012) SnapShot: bone metastasis. Cell 151 (3): 690–690 e1.
Fournier PG, Juarez P, Jiang G, Clines GA, Niewolna M, Kim HS, Walton HW, Peng XH, Liu Y, Mohammad KS, Wells CD, Chirgwin JM, Guise TA (2015) The TGF-beta signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. Cancer Cell 27 (6): 809–821.
Gong C, Maquat LE (2011) lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3' UTRs via Alu elements. Nature 470 (7333): 284–288.
Guo W, Ren D, Chen X, Tu X, Huang S, Wang M, Song L, Zou X, Peng X (2013) HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J Cell Biochem 114 (7): 1606–1615.
Hu Z, Gupta J, Zhang Z, Gerseny H, Berg A, Chen YJ, Zhang Z, Du H, Brendler CB, Xiao X, Pienta KJ, Guise T, Lee C, Stern PH, Stock S, Seth P (2012) Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. Hum Gene Ther 23 (8): 871–882.
Jakowlew SB (2006) Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev 25 (3): 435–457.
Javelaud D, Mohammad KS, McKenna CR, Fournier P, Luciani F, Niewolna M, Andre J, Delmas V, Larue L, Guise TA, Mauviel A (2007) Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res 67 (5): 2317–2324.
Juarez P, Guise TA (2011) TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone 48 (1): 23–29.
Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massague J (2005) Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 102 (39): 13909–13914.
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3 (6): 537–549.
Kunicka T, Prochazka P, Krus I, Bendova P, Protivova M, Susova S, Hlavac V, Liska V, Novak P, Schneiderova M, Pitule P, Bruha J, Vycital O, Vodicka P, Soucek P (2016) Molecular profile of 5-fluorouracil pathway genes in colorectal carcinoma. BMC Cancer 16 (1): 795.
Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, Martin P, Kelly K (2013) MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 32 (3): 296–306.
Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, Bracey JW, Hogue WR, Wong DH, Ritchie RO, Suva LJ, Derynck R, Guise TA, Alliston T (2009) Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One 4 (4): e5275.
Nakamura Y, Wood CL, Patton AP, Jaafari N, Henley JM, Mellor JR, Hanley JG (2011) PICK1 inhibition of the Arp2/3 complex controls dendritic spine size and synaptic plasticity. EMBO J 30 (4): 719–730.
Nelson WG, De Marzo AM, Isaacs WB (2003) Prostate cancer. N Engl J Med 349 (4): 366–381.
Pang Y, Young CY, Yuan H (2010) MicroRNAs and prostate cancer. Acta Biochim Biophys Sin 42 (6): 363–369.
Reichl P, Dengler M, van Zijl F, Huber H, Fuhrlinger G, Reichel C, Sieghart W, Peck-Radosavljevic M, Grubinger M, Mikulits W (2015) Axl activates autocrine transforming growth factor-beta signaling in hepatocellular carcinoma. Hepatology 61 (3): 930–941.
Ren D, Wang M, Guo W, Huang S, Wang Z, Zhao X, Du H, Song L, Peng X (2014) Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res 358 (3): 763–778.
Ren D, Wang M, Guo W, Zhao X, Tu X, Huang S, Zou X, Peng X (2013) Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR145. Int J Oncol 42 (4): 1473–1481.
Rocca DL, Amici M, Antoniou A, Blanco Suarez E, Halemani N, Murk K, McGarvey J, Jaafari N, Mellor JR, Collingridge GL, Hanley JG (2013) The small GTPase Arf1 modulates Arp2/3-mediated actin polymerization via PICK1 to regulate synaptic plasticity. Neuron 79 (2): 293–307.
Rocca DL, Hanley JG (2015) PICK1 links AMPA receptor stimulation to Cdc42. Neurosci Lett 585: 155–159.
Siegel PM, Massague J (2003) Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 3 (11): 807–821.
Son J, Park MS, Park I, Lee HK, Lee SH, Kang B, Min BH, Ryoo J, Lee S, Bae JS, Kim SH, Park MJ, Lee HS (2014) Pick1 modulates ephrinB1-induced junctional disassembly through an association with ephrinB1. Biochem Biophys Res Commun 450 (1): 659–665.
Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C, Wu J, Hu B, Cheng SY, Li M, Li J (2012) TGF-beta induces miR-182 to sustain NF-kappaB activation in glioma subsets. J Clin Invest 122 (10): 3563–3578.
Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN (1995) PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol 128 (3): 263–271.
Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL (2006) Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49 (6): 845–860.
Tang YN, Ding WQ, Guo XJ, Yuan XW, Wang DM, Song JG (2015) Epigenetic regulation of Smad2 and Smad3 by profilin-2 promotes lung cancer growth and metastasis. Nat Commun 6: 8230.
Wan X, Li ZG, Yingling JM, Yang J, Starbuck MW, Ravoori MK, Kundra V, Vazquez E, Navone NM (2012) Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 50 (3): 695–703.
Wang G, Wang Y, Feng W, Wang X, Yang JY, Zhao Y, Wang Y, Liu Y (2008) Transcription factor and microRNA regulation in androgen-dependent and -independent prostate cancer cells. BMC Genomics 9 (Suppl 2): S22.
Wang HX, Sharma C, Knoblich K, Granter SR, Hemler ME (2015) EWI-2 negatively regulates TGF-beta signaling leading to altered melanoma growth and metastasis. Cell Res 25 (3): 370–385.
Wang M, Ren D, Guo W, Huang S, Wang Z, Li Q, Du H, Song L, Peng X (2016) N-cadherin promotes epithelial-mesenchymal transition and cancer stem cell-like traits via ErbB signaling in prostate cancer cells. Int J Oncol 48 (2): 595–606.
Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW (1998) Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 77 (6): 887–894.
Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103 (2): 197–206.
Yu J, Lei R, Zhuang X, Li X, Li G, Lev S, Segura MF, Zhang X, Hu G (2016) MicroRNA-182 targets SMAD7 to potentiate TGFbeta-induced epithelial-mesenchymal transition and metastasis of cancer cells. Nat Commun 7: 13884.
Zhang B, Cao W, Zhang F, Zhang L, Niu R, Niu Y, Fu L, Hao X, Cao X (2010) Protein interacting with C alpha kinase 1 (PICK1) is involved in promoting tumor growth and correlates with poor prognosis of human breast cancer. Cancer Sci 101 (6): 1536–1542.
Zhang X, Ren D, Guo L, Wang L, Wu S, Lin C, Ye L, Zhu J, Li J, Song L, Lin H, He Z (2017) Thymosin beta 10 is a key regulator of tumorigenesis and metastasis and a novel serum marker in breast cancer. Breast Cancer Res 19 (1): 15.
Zhao B, Wang Q, Du J, Luo S, Xia J, Chen YG (2012) PICK1 promotes caveolin-dependent degradation of TGF-beta type I receptor. Cell Res 22 (10): 1467–1478.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81472505).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Dai, Y., Ren, D., Yang, Q. et al. The TGF-β signalling negative regulator PICK1 represses prostate cancer metastasis to bone. Br J Cancer 117, 685–694 (2017). https://doi.org/10.1038/bjc.2017.212
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.212
Keywords
This article is cited by
-
Identification of novel molecular subtypes to improve the classification framework of nasopharyngeal carcinoma
British Journal of Cancer (2024)
-
Membrane tension-mediated stiff and soft tumor subtypes closely associated with prognosis for prostate cancer patients
European Journal of Medical Research (2023)
-
PDIA6, which is regulated by TRPM2-AS/miR-424-5p axis, promotes endometrial cancer progression via TGF-beta pathway
Cell Death & Disease (2023)
-
Secreted miR-210-3p, miR-183-5p and miR-96-5p reduce sensitivity to docetaxel in prostate cancer cells
Cell Death Discovery (2023)
-
The long transcript of lncRNA TMPO-AS1 promotes bone metastases of prostate cancer by regulating the CSNK2A1/DDX3X complex in Wnt/β-catenin signaling
Cell Death Discovery (2023)