Abstract
Background:
Cachexia is a metabolic disorder characterised by muscle wasting, diminished response to anti-cancer treatments and poor quality of life. Our objective was to identify blood-based biomarkers of cachexia in advanced cancer patients. Hence, we characterised the plasma cytokine and blood cell mRNA profiles of patients grouped in three cohorts: patients with cachexia, pre-cachexia (no cachexia but high CRP levels: ⩾5 mg l−1) and no cachexia (no cachexia and CRP: <5 mg l−1).
Methods:
A total of 122 newly diagnosed cancer patients with seven cancer types were studied prior to their initial therapy. Plasma levels of 22 cytokines were quantified using the bio-plex technology. mRNAs isolated from whole blood and expression profiles were determined by the chip array technology and Ingenuity Pathway Analysis (IPA) software.
Results:
In comparison with non-cachectic individuals, both pre-cachectic and cachectic patients showed an increase (⩾1.5-folds) in mRNA expression of neutrophil-derived proteases (NDPs) and significantly elevated angiotensin II (Ang II) (P=0.005 and P=0.02, respectively), TGFβ1 (P=0.042 and P<0.0001, respectively) and CRP (both P<0.0001) in the plasma. Moreover, cachectic patients displayed a significant increase in IL-6 (P=0.005), IL-8 (P=0.001) and absolute neutrophil counts (P=0.007).
Conclusions:
Ang II, TGFβ1, CRP and NDP are blood biomarkers for cancer cachexia. These findings contribute to early diagnosis and prevention of cachexia.
Similar content being viewed by others
Main
Progressive muscle wasting reduces the ability of patients to perform the tasks of daily living and causes severe fatigue, leading to a major impairment in quality of life (Murphy and Lynch, 2009). It is accompanied by a reduced response to therapy, increased drug toxicity and consequent rise in morbidity and mortality. Although cachexia prevalence varies among different cancer types, it is estimated to cause up to 20% of all cancer deaths. When muscle wasting develops, it is rarely possible to reverse this deleterious cascade (Argiles et al, 2014). Muscle mass is regulated by a balance between protein synthesis and protein degradation. Loss of muscle mass is commonly observed in a variety of major chronic disease states, notably diabetes, chronic obstructive pulmonary disease, chronic heart failure, chronic renal failure and in advanced cancer. This phenomenon is associated in muscle with decreased protein synthesis and an increase in protein degradation caused by still poorly understood molecular mechanisms. A number of upstream regulators of protein degradation including growth factors (myostatin), hormones (glucocorticoids) and inflammatory cytokines promote the activation of the major catabolic systems of protein breakdown including the ubiquitin/proteasome pathway (UPP), the lysosome pathway, caspases, cathepsins and calcium-dependent calpains (McCarthy and Esser, 2010). The plasma concentration of a variety of pro-inflammatory cytokines has been analysed in numerous clinical studies of patients with cancer cachexia (Amitani et al, 2013). However, these studies do not show a clear reproducible pattern whereby the plasma concentration of any single cytokine can be correlated to the development of cachexia. Taken together, many single tumour-associated cytokines and other biomarkers have been identified; yet, none of them has served individually as a precise biomarker of cancer pre-cachexia and cachexia, impending the development of therapeutic targets, an unmet important medical need. We hypothesised that, in part, the failure of these previous studies is linked to the complex multi-faceted nature of cancer cachexia and from the fact that these studies focus on patients already presenting documented cancer cachexia. Consequently, our objectives were to examine a pre-cachexia patient cohort for changes in cytokines and transcriptomics in circulating white blood cells that could mirror early events of the wasting syndrome cascade. Such an approach has been used successfully in other diseases including in wasting syndrome associated with heart diseases (Delafontaine and Akao, 2006; Celik and Yaman, 2010; Castillero et al, 2015). We employed integrating algorithms such as Ingenuity Pathway Analysis (IPA)’ CNA (casual network analysis to identify the master upstream regulators) and URA (upstream regulator analysis to identify intermediate regulators) that provide additional levels of analysis, as not only specific biomarkers are identified but also a series of mechanistic networks and signalling pathways that are simultaneously affected by the same upstream regulators. Both IPA algorithms predict the activation or inhibition state of regulators based on downstream differentially expressed genes and known directionality of the expression. The CNA and URA algorithms include paths from regulators to regulated genes taking into account gene expression, molecular interactions or relationships based on published literature (Kramer et al, 2014). We reasoned that a comprehensive analysis of both plasma cytokine and mRNA profiles from the immune cells from pre-cachectic patients with cancer, as well as control cancer patient cohorts with no cachexia and documented cachexia, might permit the identification of specific early signatures of cancer cachexia. Herein, we analysed plasma samples of pre-cachectic patients with established inflammation but not sarcopenia in order to identify predicted blood signatures expressed. We report that neutrophil-derived proteases (NDPs) and angiotensin II (Ang II) are key master upstream regulators of cancer pre-cachexia and cachexia.
Materials and Methods
Newly diagnosed patient characteristics
Adult patients with a new diagnosis of advanced cancer, prior to receiving any oncology treatment, were recruited from the McGill University Health Centre and the Jewish General Hospital between 19 February 2009 and 22 June 2011. The description of 122 cancer patients included in the study is based on cancer type, age range, sex and disease stage (Supplementary Table 1). Patients were included if they had an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, 2 or 3, a life expectancy of at least 3 months and no symptomatic brain metastases. Ethical approval was obtained from the McGill University Research Ethics Board, and all patients and healthy subjects provided written informed consent.
Clinical data collection
Muscle mass estimation
Patients’ computed tomography scans performed for clinical purposes within 90 days of their baseline assessment were used to quantify their muscle cross-sectional area at the third lumbar (L3) vertebrae. L3 images were analysed by automated segmentation with MATLAB software (The Math Works, Inc.; Natick, MA, USA) with manual correction by an expert in musculoskeletal anatomy (Software was kindly provided by Dr Vickie Baracos and Dr Dana Cobzas, University of Alberta, Alberta, Canada). Skeletal muscle index (SMI) was calculated as muscle cross-skeletal area (cm2) per squared height (m2). A variable called mean sex-specific skeletal muscular index Z-score (z-SMI) derived from the difference between patient’s SMI and sex-specific lower limit of healthy control values (L3 SMI <55 cm2 m−2 in men and <39 cm2 m−2 in women) was created where negative values indicate sarcopenia (Mourtzakis et al, 2008). Definition of cancer cachexia: cachectic patients had >5% weight loss over the past 6 months, or ongoing weight loss of >2% with a body mass index (BMI) <20 Kg m−2, and/or sarcopenia (males <7.27 Kg m−2; females <5.45 Kg m−2 determined by dual energy X-ray absorptiometry) (Fearon et al, 2011). In this study, patients who did not meet the criteria for cachexia and had a serum C-reactive protein (CRP) ⩾5 mg l−1 were considered as pre-cachectic. This later criterion was used, because high CRP is a marker of systemic inflammatory response and one of the main factors associated with weight loss and skeletal muscle loss in patients with cancer (Douglas and McMillan, 2014). As component of GPS, CRP has equivalent or superior prognostic value compared with the subjective ECOG (Laird et al, 2013). In previous studies, CRP was also used to identify pre-cachectic patients (Argiles et al, 2011). Non-cachectic patients display CRP level <5 mg l−1. Clinical biomarkers: CRP was analysed by the McGill University Health Centre clinical laboratory and Jewish General Hospital central laboratories (normal range=0.0–5.0 mg l−1). White blood cell count (WBC) was carried out with a Beckman coulter LH750 instrument.
Cytokine profile
For the cytokine profiling, we used blood samples from 122 advanced cancer patients and 6 healthy controls. Blood was collected in EDTA-coated tubes and immediately stored at 4 °C. Within 2 h after collection, samples were processed to isolate plasma. Collection tubes were centrifuged at 2500 rpm, at 4 °C for 10 min, and the plasma was stored at −80 °C until analysis. A total of 22 cytokines were simultaneously measured in plasma. We detected IL-1β, IL-4, IL-6, IL-10, IFNγ, MCP1, IL-15, IL-8, IL-12 (p70), IL-5, IL-1α, IL-3, IL-18, TNFα, leptin, ghrelin, adiponectin, TRAIL, TGFβ1, 2, 3 with the multiplexing kits: Bio-Plex Pro Human Cytokine Group II Assay for 4 cytokines (IL-1α, IL-3, IL-18, TRAIL), Bio-Plex Pro Human Cytokine Group I Assay Group for 10 cytokines (IL-1β, IL-4, IL-6, IL-10, IFNγ, MCP1, IL-15, IL-8, IL-12 (p70), IL-5), Human cytokines (TGFβ1, 2, 3 Bio-Rad Laboratories, Hercules, CA, USA) and Leptin, Ghrelin, TNFα and Adiponectin (Millipore, Etobicoke, ON, Canada). Results were analysed using the Bio-Plex Manager 6.0 Software (Bio-rad Laboratories). Ang II levels were quantified by ELISA (Sigma Aldrich, Oakville, ON, Canada).
mRNA profile analysis
Prior to the start of any therapeutic regimen, mRNA was isolated and purified from the blood of 62 patients with low (breast cancer, 32–48%), medium (lung cancer, 54–60%) and high (pancreatic cancer, 83–85%) incidence of cachexia (Tuca et al, 2013). Whole blood (300 μl) was collected from these cancer patients at a first visit to the cancer clinic following their diagnostics. The blood was mixed with 900 μl of RNA later (Ambion, Burlington, ON, Canada) and stored at −20 °C. The RNA was purified with the RiboPure Blood Kit (Ambion). The RNA quality was first measured with a Bioanalyzer and then Affymetrix microarray assay was performed using Human Gene 1.0 ST chips. The purified RNA was sent to the Gene Expression Service Department of Genome Quebec. The analysis was performed on 1000 genes based on their reported expression in immune and cancer cells. We created two data sets, the pre-cachexia and cachexia data sets, by comparing the mRNA expression profiles of the patients with pre-cachexia and cachexia, respectively, vs those with no cachexia. Both data sets were analysed with the IPA’s CNA and URA algorithms (Qiagen, Toronto, ON, Canada). In addition, we identified the most relevant genes, whose expression increased or decreased >1.5-fold.
Statistical analysis
The mRNA profile analysis for the canonical pathways was calculated using Fisher’s exact test right-tailed and the comparisons of mRNA expression and cytokine profiles between the three different cachexia cohorts were determined with two-way ANOVA and uncorrected Fisher’s LSD (parametric distribution) and Kruskal–Wallis and Dunn’s Multiple Comparison Test (non-parametric distribution). Correlation analyses between clinical data and cytokine profile were performed with non-parametric Spearman’s correlations using Prism 6.0 Project, Graph Pad Software Inc., La Jolla, CA, USA. P-values <0.05 were considered significant.
Results
Population
We recruited 122 newly diagnosed advanced cancer patients (stages III and IV). A large proportion of patients had > 5% weight lost (46.7%), CRP level ⩾5 mg l−1 (61.5%) and sarcopenia (65.6%). About half of the population (50.8%) was classified as cachectic and 28.7% as pre-cachectic (Supplementary Table 1).
mRNA expression profile analysis of pre-cachexia and cachexia data sets with IPA
Master upstream regulators can connect through intermediate regulators to modify the gene data set target. With the use of IPA’s CNA and URA algorithms, we aim to identify regulatory networks that represent possible causes of underlying mechanisms for observed gene expression changes in the pre-cachexia and cachexia data sets. These algorithms help us predict the affected signalling pathways and the biological activities occurring in immune cells from cancer patients with different cachexia status (pre-cachexia and cachexia). The mRNA profile analysis predicts Ang II as master upstream regulator based on the downstream-regulated genes that were differently expressed between pre-cachexia and no cachexia, whereas IFNγ (P-value <0.0001), TNFα (P-value <0.0001) and IL-6 (P-value <0.0001) were identified as intermediate regulators acting downstream of Ang II. We found that these factors affect the pre-cachexia data set through the regulation of several signalling pathways including IL-8, glucocorticoid receptor (GCR), CCL5, TGFβ group, TGFβ1, Ang II, TRAIL, PTGS2 and C5 (threshold established for P-values <0.05). On the basis of the P-values, TGFβ1 and GCR were the most significant signalling pathways represented by the higher number of genes from the pre-cachexia data set (the lowest P-values indicate the highest probability of association of molecules from the pre-cachexia data set with the canonical pathway) (Figure 1A and Supplementary Tables 2–4). The mRNA profile analysis of the cachexia data set indicated that NDP cathepsins B and G were master upstream regulators (top inducers of the signalling cascade). In this case, TNFα (P-value <0.0001) and IFNγ (P-value <0.0001) were the predicted intermediate regulators acting downstream of neutrophil-derived cathepsins that affect GCR, IL-8, C5, CCL5 and TGFβ1 signalling pathways and target genes in the cachexia data set. As previously observed in the pre-cachexia data set, TGFβ1 and GCR signalling pathways were represented by higher number of genes from the cachexia data set (Figure 1B, Supplementary Tables 5–7). Genes that were commonly upregulated for the three cancer types in pre-cachexia include known genes to be expressed by neutrophils such as NDPs: elastase (ELANE, fold change pre-cachexia/no cachexia ratio or fold change=1.8), cathepsin B (CTSB, fold change=1.5) and defensins (DEFA4, fold change=3.2). We also observed upregulation of components of the nuclear factor-kappa B (NF-κB) and p38 mitogen-activated protein kinases (MAPK) pathways such as the upstream p38MAPK kinase, MKK3 (MAP2K3, fold change=1.5) and sharpin (SHARPIN, fold change=1.6, a member of linear ubiquitin chain assembly complex required for NF-kB activation (Ikeda et al, 2011) (Figure 1C and Supplementary Table 8). Similarly to pre-cachexia, the expression of MAP2K3 (cachexia/no cachexia ratio or fold change=1.4), CTSB (fold change=1.4) and SHARPIN (fold change=1.3) is consistently upregulated in cachexia. This was also true when separately examined in each of the three cancer types. Interestingly, adipsin, the additional upregulated gene in cachexia (CFD, fold change=1.5), is known to be primarily expressed by adipocytes and monocytes–macrophages in humans (Fantuzzi, 2005). As the mRNAs were collected from immune cells, the increased adipsin expression corresponds to monocyte–macrophage compartments (Figure 1D). In contrast, CD8 T-cell lineage marker (CD8A, fold change=−0.6) was the only significantly downregulated gene in the pre-cachexia and cachexia data sets, suggesting a defect of CD8 T-cell compartment (Supplementary Table 9).
Gene expression profile differences in pre-cachexia and cachexia compared with no cachexia. (A) Master upstream regulator in pre-cachexia compared with no cachexia, predicted intermediate regulators and significant signalling pathways affected in pre-cachexia (B) master upstream regulator in cachexia, predicted intermediate regulators and significant signalling pathways affected in cachexia. Heat map of common significant upregulated genes (⩾1.5-fold change) in (C) Pre-cachexia (P) and (D) Cachexia (C) compared with no cachexia (N). Data were analysed with IPA’s CNA and URA software.
Cytokine profile analysis and correlations with clinical markers
In addition to gene array studies, cytokine profile analysis from the plasma of 122 cancer patients showed the presence of higher plasma levels of TGFβ1 in patients with pre-cachexia and cachexia compared with those with no cachexia and higher levels of IL-8 and IL-6 in cachectic compared with non-cachectic patients. Only IL-8 was significant between pre-cachexia and cachexia (Figure 2A–C). In contrast, TRAIL levels decreased in cachectic compared with non-cachectic patients (Figure 2D). These results indicated that as the disease progresses to cachexia an inflammatory state is present.
Cytokine profile differences among cachexia status for all cancer types. (A) TGFβ1. (B) IL-8. (C) IL-6. (D) TRAIL. All cytokine profile comparisons were analysed using two-way ANOVA or with uncorrected Fisher’s LSD or a non-parametric test, and P-values <0.05 were considered significant. C=cachexial; P=pre-cachexia; N=no cachexia.
Validation of Ang II and neutrophil-derived cathepsins as master upstream regulators
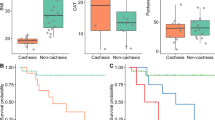
Ingenuity Pathway analysis’ CNA predicts that Ang II and NDP were the master upstream regulators of pre-cachexia and cachexia, respectively. To independently validate a potential role for Ang II, we quantified the plasma concentration of Ang II by ELISA assay from the same group of patients with lung, pancreatic and breast cancer who were categorised in the cachexia, pre-cachexia and non cachexia cohorts. Plasma levels of Ang II were significantly elevated in pre-cachectic and cachectic compared with no cachectic patients (Figure 3A). We also observed a negative correlation between Ang II and z-SMI, indicating that Ang II is associated with sarcopenia in cachectic patients (Figure 3B). Ang II levels negatively correlated with survival (Figure 3C). The absolute neutrophil count (ANC) was significantly increased in cachectic compared with no cachectic patients, and these values also negatively correlated with z-SMI (Figure 3D and E). Similarly, the NDP cathepsin B expression increased in pre-cachexia and cachexia compared with no cachexia, and, more important, its expression increased over time in the same group of pre-cachectic and cachectic patients (pancreatic, lung and breast) 3 months later after the first visit (Figure 3F). Cathepsin B expression also negatively correlated with patient’s survival (Figure 3G).
Validation of master upstream and intermediate regulators in pre-cachexia and cachexia. (A) Concentration of Ang II in the plasma. (B) Correlation between Ang II and z-SMI. (C) Correlation between plasma levels of Ang II and survival. (D) Absolute neutrophil count (ANC). (E) Correlation between ANC and z-SMI. (F) Cathepsin B expression in immune cells isolated from patients in the first visit (visit 0) and 3 months later (visit 1). (G) Correlation between cathepsin B expression and survival. Comparisons were analysed using one-way ANOVA with uncorrected Fisher’s LSD. Correlations were analysed using non-parametric Spearman’s correlation two tailed. P-values <0.05 were considered significant. z-SMI: derived from the difference between patient’s Skeletal Muscle Index and sex-specific lower limit of healthy control values (L3 SMI <55 cm2 m−2 in men and <39 cm2 m−2 in women) where negative values indicate sarcopenia. C=cachexia; P=pre-cachexia; N=no cachexia.
Haematologic and biochemical pronostic variables in cachexia
We also evaluated neutrophil/lymphocyte ratios, albumin levels, CRP, LDH concentration, absolute lymphocyte count (ALC) and WBC. Similarly to ANC, the neutrophil/lymphocyte ratio was significantly elevated in cachexia compared with no cachexia (Figure 4A). In contrast, the ALC was not different in the cachexia cohort (data not shown). We also observed significant lower albumin levels and increased levels of CRP in both pre-cachectic and cachectic patients (Figure 4B and C). In contrast, no meaningful differences were detected with regard to LDH concentrations and WBC (data not shown).
Quantification of indicators of inflammation and cancer progression. (A) Absolute number of neutrophil/lymphocyte ratio. (B) CRP plasma levels. (C) Albumin plasma levels. Comparisons were analysed using one-way ANOVA with uncorrected Fisher’s LSD or a non-parametric test. P-values <0.05 were considered significant. C=cachexia; P=pre-cachexia; N=no cachexia.
Correlation analysis between cytokine profile and the clinical indicators of cachexia
The three clinical markers of cachexia (BMI, weight loss, CRP and sarcopenia) were correlated with the plasma levels of 22 cytokines and metabolomic hormones. In pre-cachexia patients, TRAIL plasma levels negatively correlated with CRP values (Figure 5A). In cachexia patients, only TNFα correlated with CRP values (Figure 5B), whereas TNFα and IL-8 levels correlated with sarcopenia (negative z-SMI values) (Figure 5C and D).
Discussion
Seely has described the biologic complex non linear systems as ‘ a system or whole consisting of an extremely large and variable number of component parts where the individual components display marked variability over time, and are characterised by a high degree of connectivity or interdependence between variables’. The Anorexia Cachexia Syndrome displays the characteristics of such a system. One can hypothesise that it varies from patient to patient and even, over time, within the same patient. Among other variables, cytokine pleiotropy, constantly changing feedback loops and inconsistent autonomic influences are likely present. We report on two variables that we believe are important ingredients of the cachexia stew in cancer patients. In our studies, we specifically accrued newly diagnosed patients with advanced cancers. Using blood samples obtained from these individuals prior to any anti-cancer therapy, we, respectively, profiled plasma cytokines and gene array, which led to our findings of an important interplay between Ang II and neutrophil proteases in both cancer-related inflammation and cachexia. This interaction is evident in patients before weight loss is apparent. The recognition of this early phase that we associated with the pre-cachectic state uncovered a cascade of signalling events, leading to the wasting syndrome that appears much prior to the presence of weight loss.
Angiotensin II
As master upstream regulator of pre-cachexia, increased plasma levels of Ang II correlated with sarcopenia and decreased patient’s survival. Ang II can promote cachexia through several mechanisms. Ang II promotes actin cleavage, proteolysis and increased apoptosis by inhibiting phospho-Akt and activating caspase-3 in skeletal muscle (Song et al, 2005). In addition, Ang II was shown to inhibit protein synthesis by lowering IGF-1 in skeletal muscle (Trobec et al, 2011). Indirectly, Ang II promotes muscle atrophy by increasing the levels of circulating cytokines and hormones including glucocorticoids, IL-8, IL-6 and TNFα, which also promote muscle protein degradation. In addition, Ang II reduces the appetite by acting directly on hypothalamic neurons to regulate orexigenic/anorexigenic neuropeptides (Fearon et al, 2012; Vogiatzi et al, 2013; Yoshida et al, 2013). As possible therapeutic intervention, the inhibition of the renin–angiotensin system may improve the clinical outcome in cachectic patients and consequently their quality of life and response to the current cancer therapies. Previous studies demonstrate that the inhibition of Ang II with the angiotensin-converting enzyme inhibitor prevents protein degradation in skeletal muscle and improves weight loss (Sanders et al, 2005; Yoshida et al, 2013).
The contribution of neutrophils and their derived proteases to cachexia
Higher mRNA expression of NDP, along with increased ANC observed in pre-cachexia and cachexia, suggests that activated neutrophils and their products may trigger a cascade of events required for the progression toward cachexia. A mechanism of neutrophil-mediated muscle injury appears to be directly dependent on CD18-mediated neutrophil adhesion to myotubes and iron-dependent hydroxyl radical production (McLoughlin et al, 2003). Furthermore, NDP such as cathepsin B can release Ang II from Ang I and angiotensinogen (Ramaha and Patston, 2002; Vidotti et al, 2004) and induce insulin receptor substrate-1 degradation, lower insulin signalling and increase glucose production in primary mouse and human hepatocytes (Talukdar et al, 2012). In accordance with these studies, we observed a significant increase in cathepsin B expression over time in pre-cachectic and cachectic patients and a negative correlation between cathepsin B expression and survival. Together, the chronic elevation of neutrophils and its proteases in the blood of cancer patients provides a concerted ‘cocktail’ of proteases and cytokines that may drive the wasting syndrome.
GCR, TGFβ1 and IL-8
In agreement with the mRNA profile analysis, the cytokine profile shows increased plasma levels of Ang II and TGFβ1 in pre-cachectic and cachetic patients. Ang II regulates several intracellular signalling pathways including TGF-β/Smads, NF-κB, glucocorticoids and mitogen-activated protein kinases (Moreno et al, 2009). We found that GCR and TGFβ1 are the most represented significant signalling pathways in both pre-cachexia and cachexia data sets. It is well known that glucocorticoids promote muscle atrophy by inducing the activation of UPP and by suppressing the insulin/IGF-1 signalling (Hu et al, 2009). Ang II stimulates TGFβ1 expression, promotes its conversion to bioactive TGFβ1 and upregulates TGFβ receptors (Wolf, 2006). Both glucocorticoids and TGFβ1 preferably induce CD8 T-cell apoptosis (Lanza et al, 1996; Bhadra et al, 2014). Thus, the marked decrease in CD8A gene expression observed in pre-cachectic and cachectic patients could be due to severe immunosuppressive effects of glucocorticoids and active TGFβ1 on this immune cell compartment. IL-8 is significantly increased in cachexia compared with pre-cachexia and no cachexia. IL-8 amplifies the inflammatory cascade by promoting the recruitment and degranulation of neutrophils releasing their proteases to the circulation. IL-8 has been reported to be associated with involuntary weight loss in oesophageal and gastric cancer patients (Krzystek-Korpacka et al, 2007).
IL-6, TNFα, TRAIL and albumin levels
As previously published (Narsale and Carson, 2014), IL-6 increases in cachexia. Circulating levels of this pro-inflammatory cytokine have been shown to correlate with weight loss and reduced survival in pancreatic cancer patients. IL-6 stimulates the liver to produce acute-phase response proteins (Moses et al, 2009). Although the TNFα level was not significant among the cachexia status, it shows a negative correlation with z-SMI and a positive correlation with CRP in cachectic patients. We identified a reduction in TRAIL levels in cachectic patients, as well as a negative correlation with CRP in pre-cachectic patients. It has been reported that TRAIL has a protective role in the immune surveillance against tumours (Di Pietro and Zauli, 2004). Recent studies have shown that elevated CRP levels during systemic inflammation in cancer patients contribute to tumour progression by downregulating TRAIL expression in immune cells (Secchiero et al, 2013). We also observed a significant decrease in albumin levels in pre-cachectic and cachectic patients. As it was recently reported, low albumin levels along with weight loss, fatigue and high CRP levels predict shorter survival in cancer patients (Op den Kamp et al, 2012).
The role of Ang II and NDP
We propose that Ang II and NDP are among the major molecular mechanisms that drive cancer cachexia. As tumour grows, it provokes tissue damage, necrosis and hypoxia that release a number of chemoattracting factors favouring the recruitment and activation of neutrophil and macrophages, which in turn secrete IL-8, a potent neutrophil-chemoattracting factor. Activated neutrophils release proteases to the circulation that generate Ang II from Ang I and angiotensinogen. Elevated plasma levels of Ang II and Ang II-mediated production of IL-6, IL-8 and TGFβ1 will contribute to the inflammation state and cause induction of protein degradation and/or inhibition of protein synthesis. The plasma concentration of these blood biomarkers markedly increases in pre-cachectic and cachectic patients (Table 1), in which case muscle protein degradation and wasting are irriversable. Our clinical study offers a detailed description of a cellular and molecular cascade present in patients with cancer cachexia. This cascade is driven by the interplay of the Ang II peptide hormone in the circulation and the rise in NDP. We found that it is already activated in patients presenting chronic inflammation but without cachexia. In conclusion, our study suggests that efforts to ease unbridled neutrophil activity and Ang II are worthwhile approaches to reduce or prevent the appearance of cancer cachexia.
Change history
15 March 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Amitani M, Asakawa A, Amitani H, Inui A (2013) Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol 45 (10): 2179–2185.
Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ (2014) Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 14 (11): 754–762.
Argiles JM, Lopez-Soriano FJ, Toledo M, Betancourt A, Serpe R, Busquets S (2011) The cachexia score (CASCO): a new tool for staging cachectic cancer patients. J Cachexia Sarcopenia Muscle 2 (2): 87–93.
Bhadra R, Moretto MM, Castillo JC, Petrovas C, Ferrando-Martinez S, Shokal U, Leal M, Koup RA, Eleftherianos I, Khan IA (2014) Intrinsic TGF-beta signalling promotes age-dependent CD8+T cell polyfunctionality attrition. J Clin Invest 124 (6): 2441–2455.
Castillero E, Akashi H, Wang C, Najjar M, Ji R, Kennel PJ, Sweeney HL, Schulze PC, George I (2015) Cardiac myostatin upregulation occurs immediately after myocardial ischemia and is involved in skeletal muscle activation of atrophy. Biochem Biophys Res Commun 457 (1): 106–111.
Celik T, Yaman H (2010) Elevated adiponectin levels in patients with chronic heart failure: an independent predictor of mortality or a marker of cardiac cachexia? Int J Cardiol 144 (2): 319–320.
Delafontaine P, Akao M (2006) Angiotensin II as candidate of cardiac cachexia. Curr Opin Clin Nutr Metab Care 9 (3): 220–224.
DiPietro R, Zauli G (2004) Emerging non-apoptotic functions of tumor necrosis factor-related apoptosis-inducing ligand(TRAIL)/Apo2L. J Cell Physiol 201 (3): 331–340.
Douglas E, McMillan DC (2014) Towards a simple objective framework for the investigation and treatment of cancer cachexia: the Glasgow Prognostic Score. Cancer Treat Rev 40 (6): 685–691.
Fantuzzi G (2005) Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115 (5): 911–919, quiz 920.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12 (5): 489–495.
Fearon KC, Glass DJ, Guttridge DC (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16 (2): 153–166.
Hu Z, Wang H, Lee IH, Du J, Mitch WE (2009) Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest 119 (10): 3059–3069.
Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, vanWijk SJ, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I (2011) SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature 471 (7340): 637–641.
Kramer A, Green J, PollardJ Jr., Tugendreich S (2014) Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30 (4): 523–530.
Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, Banas T (2007) Impact of weight loss on circulating IL-1, IL-6, IL-8, TNF-alpha, VEGF-A, VEGF-C and midkine in gastroesophageal cancer patients. Clin Biochem 40 (18): 1353–1360.
Laird BJ, Kaasa S, McMillan DC, Fallon MT, Hjermstad MJ, Fayers P, Klepstad P (2013) Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res 19 (19): 5456–5464.
Lanza L, Scudeletti M, Puppo F, Bosco O, Peirano L, Filaci G, Fecarotta E, Vidali G, Indiveri F (1996) Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol 103 (3): 482–490.
McCarthy JJ, Esser KA (2010) Anabolic and catabolic pathways regulating skeletal muscle mass. Curr Opin Clin Nutr Metab Care 13 (3): 230–235.
McLoughlin TJ, Tsivitse SK, Edwards JA, Aiken BA, Pizza FX (2003) Deferoxamine reduces and nitric oxide synthase inhibition increases neutrophil-mediated myotube injury. Cell Tissue Res 313 (3): 313–319.
Moreno M, Ramalho LN, Sancho-Bru P, Ruiz-Ortega M, Ramalho F, Abraldes JG, Colmenero J, Dominguez M, Egido J, Arroyo V, Gines P, Bataller R (2009) Atorvastatin attenuates angiotensin II-induced inflammatory actions in the liver. Am J Physiol Gastrointest Liver Physiol 296 (2): G147–G156.
Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA (2009) Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep 21 (4): 1091–1095.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33 (5): 997–1006.
Murphy KT, Lynch GS (2009) Update on emerging drugs for cancer cachexia. Expert Opin Emerg Drugs 14 (4): 619–632.
Narsale AA, Carson JA (2014) Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care 8 (4): 321–327.
Op den Kamp CM, Langen RC, Minnaard R, Kelders MC, Snepvangers FJ, Hesselink MK, Dingemans AC, Schols AM (2012) Pre-cachexia in patients with stages I-III non-small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer 76 (1): 112–117.
Ramaha A, Patston PA (2002) Release and degradation of angiotensin I and angiotensin II from angiotensinogen by neutrophil serine proteinases. Arch Biochem Biophys 397 (1): 77–83.
Sanders PM, Russell ST, Tisdale MJ (2005) Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role incancer cachexia. Br J Cancer 93 (4): 425–434.
Secchiero P, Rimondi E, diIasio MG, Agnoletto C, Melloni E, Volpi I, Zauli G (2013) C-Reactive protein downregulates TRAIL expression in human peripheral monocytes via an Egr-1 dependent pathway. Clin Cancer Res 19 (8): 1949–1959.
Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P (2005) Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 115 (2): 451–458.
Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM (2012) Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med 18 (9): 1407–1412.
Trobec K, von Haehling S, Anker SD, Lainscak M (2011) Growth hormone, insulin-like growth factor 1, and insulin signaling-a pharmacological target in body wasting and cachexia. J Cachexia Sarcopenia Muscle 2 (4): 191–200.
Tuca A, Jimenez-Fonseca P, Gascon P (2013) Clinical evaluation and optimal management of cancer cachexia. Crit Rev Oncol Hematol 88 (3): 625–636.
Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA (2004) High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286 (6): F1039–F1045.
Vogiatzi K, Apostolakis S, Vlata Z, Krabovitis E, Spandidos DA (2013) Opposite effect of angiotensin receptor blockade on CXCL8 production and CXCR1/2 expression of angiotensin II treated THP-1monocytes. Exp Ther Med 5 (3): 987–991.
Wolf G (2006) Renal injury due to renin-angiotensin-aldosterone system activation of thet ransforming growth factor-beta pathway. Kidney Int 70 (11): 1914–1919.
Yoshida T, Tabony AM, Galvez S, Mitch WE, Higashi Y, Sukhanov S, Delafontaine P (2013) Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. Int J Biochem Cell Biol 45 (10): 2322–2332.
Acknowledgements
We acknowledged the Terry Fox Research Institute (TFRI) to the funding of this study. CP is a fellow of the CIHR. This research was supported by a grant from the TFRI and by the Cancer Research Society CRS/Rob Lutterman Pancreatic Cancer Research Grant. BG is a recipient of ‘Chercheur-clinicien Boursier’ award from FRQS, Québec, Canada. MLT is the holder of the Jeanne and Jean-Louis Levesque Chair in Cancer Research at McGill University. Finally, we thank all patients and their families who have made this study possible. The corresponding authors confirm that they had full access to all the data in the study and the final responsibility for the decision to submit for publication. We are grateful to Professor Vickie Baracos for helpful suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Penafuerte, C., Gagnon, B., Sirois, J. et al. Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br J Cancer 114, 680–687 (2016). https://doi.org/10.1038/bjc.2016.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.3
Keywords
This article is cited by
-
Cancer- and cardiac-induced cachexia: same fate through different inflammatory mediators?
Inflammation Research (2022)
-
Prognostic value of an inflammation-based nutritional score for patients with initially unresectable pancreatic adenocarcinoma undergoing conversion surgery following chemo-/radiotherapy
Surgery Today (2021)
-
Serum anti-AP3D1 antibodies are risk factors for acute ischemic stroke related with atherosclerosis
Scientific Reports (2021)
-
An elevated neutrophil-to-lymphocyte ratio associates with weight loss and cachexia in cancer
Scientific Reports (2020)
-
Immunohistochemical phenotyping of T cells, granulocytes, and phagocytes in the muscle of cancer patients: association with radiologically defined muscle mass and gene expression
Skeletal Muscle (2019)