Abstract
Background:
131I-metaiodobenzylguanidine (MIBG) is an active radiopharmaceutical in neuroblastoma. A previous study demonstrated that MIBG could be combined with vincristine and prolonged irinotecan, although 25% of first courses had grade 3 diarrhoea. The current phase I/II study evaluated MIBG with vincristine and 5 days of higher-dose irinotecan.
Methods:
Patients 1–30 years old with advanced neuroblastoma were eligible. Patients received cefixime on days −1 to +6, irinotecan (50 mg m−2 per dose IV) on days 0–4, vincristine (2 mg m−2) on day 0, MIBG (555 or 666 MBq kg−1) on day 1, and peripheral blood stem cells on day 13. UGT1A1 genotyping was performed in consenting patients.
Results:
Thirty-two patients (12 phase I ; 20 phase II) received 42 courses. No dose-limiting toxicities were seen during dose escalation and the recommended administered activity was 666 MBq kg−1. Myelosuppression and diarrhoea were the most common toxicities, with grade 3 diarrhoea in 6% of first courses. Patients homozygous for UGT1A1*28 had more grade 4 thrombocytopenia (80% vs 37%; P=0.14). Responses (five complete and four partial) occurred in 9 out of 32 (28%) patients.
Conclusions:
MIBG (666 MBq kg−1) with vincristine and this irinotecan schedule is tolerable and active, with less severe diarrhoea compared with a regimen using more protracted irinotecan.
Similar content being viewed by others
Main
Neuroblastoma is a paediatric malignancy derived from the sympathetic nervous system. Approximately 90% of neuroblastoma tumours accumulate metaiodobenzylguanidine (MIBG), a norepinephrine analogue (Treuner et al, 1984). MIBG radiolabelled with 131I (131I-MIBG) is used as a systemic targeted radiopharmaceutical that has an important role in the treatment of patients with relapsed or refractory neuroblastoma (DuBois and Matthay, 2008). As a single agent, 131I-MIBG has one of the highest response rates in this setting, with a manageable toxicity profile (DuBois et al, 2004; Matthay et al, 2007).
More recent efforts have focused on strategies to improve 131I-MIBG therapy, either by using it earlier in the course of the disease or by combining it with systemic radiation sensitisers. Topotecan has been shown preclinically to increase the antitumour activity of 131I-MIBG, and a small pilot study demonstrated the feasibility of combining 131I-MIBG with topotecan in children with advanced neuroblastoma (Gaze et al, 2005; McCluskey et al, 2005). As North American induction chemotherapy already utilises topotecan (Park et al, 2011), our group has instead studied another camptothecin, irinotecan, as a radiation sensitiser in combination with 131I-MIBG. In an initial phase I clinical trial (NANT04-06), we demonstrated that vincristine and irinotecan could be combined with 131I-MIBG at its usual maximum feasible dose of 666 MBq kg−1 (18 mCi kg−1; DuBois et al, 2012). The response rate of 25% was promising in the context of a phase I trial. The irinotecan regimen in NANT04-06 prescribed irinotecan 20 mg m−2 per day × 5 days per week for two consecutive weeks. This regimen was selected for two reasons. First, it was commonly used in paediatric oncology at the time (Wagner et al, 2004; Bagatell et al, 2011). Second, radiation exposure following a single administration of 131I-MIBG lasts for several weeks, as the 131I decays with a physical half-life of 8 days. This prolonged irinotecan schedule provided extensive overlap between the radiation sensitiser and this radiation exposure.
Evaluation of the toxicity data from NANT04-06 showed that 25% of 24 first courses resulted in grade 3 diarrhoea. This toxicity not only had an impact on patients, but potentially also on nurses and caregivers caring for patients in radiation isolation. The toxicity profile of irinotecan is schedule-dependent, with greater haematologic toxicity with higher-dose shorter-exposure regimens and greater gastrointestinal toxicity with lower-dose more prolonged exposure regimens (O’Leary and Muggia, 1998). Therefore, we hypothesised that using a higher-dose shorter-exposure regimen could reduce the risk of diarrhoea from the combination of 131I-MIBG with vincristine and irinotecan. However, this change might increase the risk of myelosuppression and reduce the efficacy of the combination, by reducing the degree of overlap between the radiation sensitiser and the radiation. To assess these issues, we conducted a phase I/II clinical trial of a new regimen of 131I-MIBG with vincristine and short-course irinotecan. The role of vincristine in this combination is not clear, as it is not thought to be a radiation sensitiser. In another paediatric solid cancer, vincristine was synergistic when added to irinotecan (Pappo et al, 2007). Therefore, vincristine was incorporated into the prior regimen we tested. In order to keep the regimen as constant as possible except for the desired change in irinotecan, we included vincristine in the current regimen as well. As the risk of irinotecan-associated myelosuppression correlates with UGT1A1 genotype in adults (Deeken et al, 2008), we included a correlative pharmacogenomics aim to assess this issue in paediatrics.
MATERIALS AND METHODS
Patient eligibility
Patients with high-risk neuroblastoma were eligible if they were 1–30 years of age and had relapsed/progressive disease, less than a partial response to induction therapy, partial response to induction but at least three residual lesions on end-induction MIBG scan or partial response to induction therapy with biopsy-proven residual disease. All patients were required to have MIBG-avid disease based on diagnostic imaging obtained within 4 weeks of enrolment and subsequent to intervening therapy. All patients had ⩾2.0 × 106 CD34+ autologous haematopoietic stem cells kg−1, Lansky or Karnofsky performance score ⩾50 and estimated life expectancy ⩾6 weeks. There was no restriction on the number of lines of prior therapy received before enrolment. Patients were allowed to have received prior 131I-MIBG therapy as long as at least 6 months had elapsed since last 131I-MIBG therapy, the 131I-MIBG had not been in combination with irinotecan and the cumulative lifetime dose did not exceed 18 mCi kg−1. Other required minimum washout periods were as follows: 2 weeks for systemic therapy; 3 months for autologous stem cell transplant; 2 weeks for small port radiation; and 3 months for large field radiation. Patients were excluded if they had prior whole-abdominal or total body radiation, or allogeneic transplantation.
All patients met the following organ function requirements: unsupported absolute neutrophil count (ANC) ⩾750 mm−3; unsupported platelet count ⩾50 000 mm−3; haemoglobin ⩾8 g dl−1; creatinine clearance ⩾60 ml min−1 per 1.73 m2 or serum creatinine ⩽1.5 times the upper limit for age-adjusted normal value; total bilirubin ⩽1.5 times the upper limit; ALT and AST ⩽3 times the upper limit; left ventricular ejection fraction ⩾55% or shortening fraction ⩾27%; and normal pulmonary function as defined by the lack of dyspnoea at rest, exercise intolerance, pleural effusion or oxygen requirement.
Patients were excluded for any of the following: pregnancy/breast feeding; other major concurrent illnesses; concomitant enzyme-inducing anticonvulsants, ketoconazole, or St John’s wort; need for haemodialysis; cephalosporin allergy; diarrhoea ⩾grade 2; and active infection.
The institutional review board of each site approved the study. All patients and/or legal guardians provided written informed consent for participation, with assent obtained as appropriate.
Treatment
Day −1 was defined as the start of the protocol therapy and was to occur within 2 weeks of enrolment. To reduce the risk of diarrhoea, all patients received cefixime or cefpodoxime orally on days −1 to +6 (Wagner et al, 2008). Patients received vincristine (2 mg m−2 per dose to the maximum dose of 2 mg) as an intravenous bolus on day 0. Patients received irinotecan (50 mg m−2 per dose to the maximum dose of 100 mg) as an intravenous infusion over 60–90 min on days 0–4.
131I-MIBG was infused intravenously over 90–120 min on day 1 at either 555 MBq kg−1 (15 mCi kg−1) or 666 MBq (18 mCi kg−1), with a maximum absolute administered activity of 44 400 MBq (1200 mCi). 131I-MIBG was obtained from Jubilant DraxImage Inc. (Kirkland, Quebec, Canada), with a specific activity ⩾1098.9 MBq mg−1 MIBG and maximum allowable free iodine content <5%. Patients remained in radiation isolation until they met local radiation safety requirements. The use of an indwelling urinary catheter and thyroid blockade were standard and as previously described (Matthay et al, 2007), except potassium perchlorate was no longer available for use during this study. Estimated whole-body dose received was estimated as previously described (Matthay et al, 2001).
Patients received a minimum of 2.0 × 106 CD34+ cells kg−1 on day 13. They received filgrastim or pegfilgrastim if ANC decreased to <500 mm−3, with filgrastim continuing until ANC recovered to >2000 mm−3.
Patients were eligible to receive a second course at least 42 days after the first 131I-MIBG infusion if they had at least stable disease, had recovered to baseline criteria, had no dose-limiting toxicity (DLT; see next section) in the first course; had PBSCs available to support a second course, and would not exceed a lifetime cumulative maximum 131I-MIBG dose of 1332 MBq kg−1 (36 mCi kg−1) with the second course.
In the phase I portion of the study, all patients were treated at the UCSF Benioff Children’s Hospital. In the phase II portion of the study, patients were treated either at UCSF or at the Children’s Hospital of Philadelphia.
Toxicity and response evaluation
Toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0. Non-haematologic DLT was defined as grade ⩾3 toxicity with the exception of the following grade 3 toxicities: nausea; vomiting; dehydration; anorexia; febrile neutropenia or infection; fever; electrolyte abnormality; or hepatic enzyme or amylase elevation returning to ⩽grade 1 by day 56. Grade 3 diarrhoea was considered dose-limiting only if it persisted for more than 72 h despite maximal supportive care measures. Haematologic DLT was defined as: grade 4 neutrophils 28 days after PBSC infusion; platelets <20 000 mm−3 56 days after PBSC infusion; need for a second PBSC infusion; grade 4 haemolysis; refractory to platelet transfusions with life-threatening bleeding; or life-threatening anaemia. Any DLT needs to be deemed at least possibly related to the protocol therapy.
Patients underwent disease staging within 4 weeks of the study entry and then ∼6 weeks following 131I-MIBG. Response was graded using the same NANT response criteria used for NANT04-06 (DuBois et al, 2012). These criteria utilise RECIST 1.0 criteria for measurable tumours (Therasse et al, 2000), Curie score for MIBG scan response (Ady et al, 1995) and bone marrow (BM) morphology to define response across disease sites. Overall response to one course of therapy could be no better than the worst response at each of these disease sites. All MIBG scans and all CT/MRI scans were centrally reviewed. All BM reports were centrally reviewed; however, slides were not re-reviewed. Patients with an overall best response of complete or partial response were considered to have responded, and all other patients were considered non-responders.
Irinotecan pharmacogenomics
Patients consenting to an optional irinotecan pharmacogenomics study provided a single blood sample at study entry. DNA was extracted from mononuclear cells using the standard methods and then UGT1A1 polymorphisms were genotyped using the standard techniques in the UCSF Clinical Laboratory.
Statistical methods
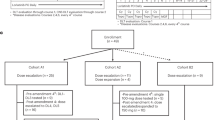
In this phase I/II study, a limited dose-escalation phase included two dose levels (555 MBq kg−1 (15 mCi kg−1) and 666 MBq kg−1 (18 mCi kg−1)) evaluated according to a modification of the rolling 6 design (Skolnik et al, 2008). Six patients were to enrol at 555 MBq kg−1 (15 mCi kg−1) and if less than two of the six had protocol-defined first-course DLT, evaluation of the six patients at 666 MBq kg−1 (18 mCi kg−1) was to proceed. If less than two of the six had protocol-defined first-course DLT at 666 MBq kg−1 (18 mCi kg−1), the phase II portion was to proceed with this administered activity. The protocol initially planned to enrol an additional 24 patients in the phase II portion to provide a planned total of 30 patients treated with 666 MBq kg−1 (18 mCi kg−1). Owing to the activation of a competing randomized phase II trial, the protocol closed early after 20 additional patients enrolled to the phase II portion, providing 26 total patients who were treated at this administered activity level. Exact confidence intervals around response rates were calculated as previously described (Clopper and Pearson, 1934). The Fisher exact test was used to compare rates of adverse events according to the UGT1A1 genotype. The Wilcoxon rank sum test was used to compare whole-body radiation dose between groups defined by response and toxicity.
Results
Patient characteristics
Thirty-two patients enrolled and received 42 courses of therapy. All were eligible and all were evaluable for toxicity and response. Characteristics of the 32 patients are shown in Table 1. Exactly half of the patients were treated for relapsed disease, whereas the remaining half were treated for inadequate response to initial therapy. Only two patients had received prior 131I-MIBG therapy, although 18 patients had received prior irinotecan therapy. Patients typically had extensive disease involvement at study entry, with a median Curie score of 11 and 63% of patients with BM involvement at entry.
Dose escalation and toxicity
Six patients enrolled and were treated at the 555 MBq kg−1 (15 mCi kg−1) dose level. None of the patients had protocol-defined DLT. Six patients then enrolled and were treated at the 666 MBq kg−1 (18 mCi kg−1) dose level and none had protocol-defined DLT. Therefore, enrolment to the phase II portion proceeded at the 666 MBq kg−1 (18 mCi kg−1) dose level. Twenty additional patients enrolled to this dose level, making a total of 26 patients treated with 666 MBq kg−1 (18 mCi kg−1). The median estimated whole-body radiation doses were 192 cGy (range 145–271) in six patients with available data at 555 MBq kg−1 (15 mCi kg−1) and 254 cGy (range 140–441) in 21 patients with available data at 666 MBq kg−1 (18 mCi kg−1). Dosimetry data were missing in five patients because of incomplete or absent data collection. The median total caregiver exposure for the 29 patients with available first-course data was 1.15 mSv (range 0.015–2.47 mSv).
Details of non-haematologic toxicity by dose level are shown in Table 2. There were no grade 4 or 5 non-haematologic toxicities and, at dose level 555 MBq kg−1 (15 mCi kg−1), there were no reported grade 3 non-haematologic toxicities. At dose level 666 MBq kg−1 (18 mCi kg−1), grade 3 vomiting occurred in 11% of first courses and all other grade 3 non-haematologic toxicities occurred in <10% of first courses. Across dose levels, 69% of patients had grade 1–2 and 6% of patients had grade 3 first-course diarrhoea. Whole-body dose did not differ between patients with and without diarrhoea (data not shown).
Details of first-course haematologic toxicity by the dose level are shown in Table 3. First-course haematologic toxicity was observed in all patients, including 100% of patients with neutropenia and 100% of patients with thrombocytopenia of any grade. Grade 4 neutropenia was seen in 83% of patients treated with 555 MBq kg−1 (15 mCi kg−1) and in 81% of patients treated with 666 MBq kg−1 (18 mCi kg−1). All patients with grade 4 neutropenia engrafted within 28 days from stem cell infusion (median of 10 days at 555 MBq kg−1 (15 mCi kg−1) and 8 days at 666 MBq kg−1 (18 mCi kg−1)). Grade 4 thrombocytopenia was seen in 17% of patients at 555 MBq kg−1 (15 mCi kg−1) and 58% of patients at 666 MBq kg−1 (18 mCi kg−1). All patients with grade 4 thrombocytopenia engrafted within 56 days (18 days for the one patient at 555 MBq kg−1 (15 mCi kg−1) and the median of 14 days for patients at 666 MBq kg−1 (18 mCi kg−1)). Whole-body dose did not differ between patients with and without grade 4 neutropenia or grade 4 thrombocytopenia (data not shown).
In terms of late effects of protocol therapy, three patients developed grade 1 or 2 hypothyroidism, all in the 666 MBq kg−1 (18 mCi kg−1) group. One patient developed fatal myelodysplastic syndrome transforming to acute myeloid leukaemia 10.5 months after one course of 131I-MIBG therapy at the 666 MBq kg−1 (18 mCi kg−1) dose level on this study. The patient had received multiple prior treatment regimens, including prior therapy with 131I-MIBG on a different clinical trial.
Responses
Table 4 shows responses according to the dose level and site of disease involvement. The overall best response rate across both dose levels after a maximum of two courses was 28% (five complete and four partial responses; 95% confidence interval 14–47%). The overall best response rate was 48% by MIBG diagnostic scan criteria, 33% by BM evaluation and 20% by CT scan. There was some association between whole-body dose after the first course and response to the first course. Specifically, the median whole-body dose for first-course responders (n=5) was 327 cGy (range 189–400 cGy) vs 232 cGy (range 140–441 cGy; P=0.06 by Wilcoxon rank sum test) for non-responders (n=22).
Among the six patients treated with 555 MBq kg−1 (15 mCi kg−1), the overall best response rate was 50%. Among the 26 patients treated with 666 MBq kg−1 (18 mCi kg−1), the overall best response rate was 23% (95% confidence interval 9–44%). Had the study completed full enrolment to 30 patients at this dose level and the response rate remained the same, then the 95% confidence interval would have narrowed slightly to 10–42%.
Ten patients were treated with two courses of therapy. In four of these patients, the best response occurred following the second course of therapy. No patients with at least stable disease after the first course of therapy developed progressive disease after the second course of therapy.
Of the two patients who were treated with prior 131I-MIBG, one patient had a partial response to re-treatment on this study and one patient had stable disease as their best response. Four of eighteen (22%) patients treated with prior irinotecan had an overall objective response, similar to that seen in the overall study.
Irinotecan pharmacogenomics
The UGT1A1 genotype was determined for 24 patients (Table 5). The UGT1A1 genotype was not associated with risk of diarrhoea. In fact, the two patients with diarrhoea >grade 2 and available genotype data were homozygous for the wild-type allele. No association was found between the UGT1A1 genotype and risk of neutropenia. Patients homozygous for UGT1A1*28 appeared to be at increased risk for grade 4 thrombocytopenia, although the association was not statistically significant (80% for homozygous UGT1A1*28 vs 37% for all other genotypes; P=0.14).
Discussion
Our results demonstrate that 131I-MIBG can be administered at its usual maximum feasible administered activity of 666 MBq kg−1 (18 mCi kg−1) along with vincristine and a higher dose, shorter course of irinotecan. Consistent with our hypothesis that motivated this new combination, the rates of grade 3 diarrhoea were lower with this combination than with our previous trial using a lower-dose prolonged administration schedule (DuBois et al, 2012). Caregiver exposures were similar to levels previously reported (Gains et al, 2014). Despite having less overlap between the putative radiation sensitiser and radiation compared with our prior study, the antitumour activity was similar between the two trials (28% response rate in the current study vs 25% in the prior study). We note that the early closure of the study because of the activation of a competing trial resulted in a slight increase in the confidence interval around the point estimate in the response rate, but did not alter the conclusion of a similar response rate to the prior study. The UGT1A1 genotype correlated with risk of thrombocytopenia, although this finding was not statistically significant in the context of this phase I/II trial.
Several aspects of the toxicity profile of this regimen are noteworthy. As stated, the rates of first-course grade 3 diarrhoea were lower with this regimen compared with our prior regimen (6% in the current study vs 25%). Interestingly though, the rates of first-course diarrhoea of any grade were similar between the two studies (69% in the current study vs 75%). While any diarrhoea during clearance of the radiation associated with 131I-MIBG is undesirable, the lower rates of severe diarrhoea with this new regimen improve the safety and tolerability of this combination. In the largest study to date of single agent 131I-MIBG, no cases of grade 3 or higher diarrhoea were reported in 164 administrations (Matthay et al, 2007). Therefore, while the current combination regimen has low rates of grade 3 diarrhoea, it nevertheless appears to carry a higher risk of severe diarrhoea compared with 131I-MIBG alone.
Given the new irinotecan-dosing schedule, we expected higher rates of myelosuppression with this new regimen. Rates of severe thrombocytopenia were similar between patients treated on this study and patients treated at 555 MBq kg−1 (15 mCi kg−1) or 666 MBq kg−1 (18 mCi kg−1) dose levels on the prior study (47% vs 50%, respectively). Rates of grade 4 neutropenia were somewhat higher on the current regimen (81% vs 61%, respectively), although infectious complications were uncommon. The use of routine prophylactic stem cell support in both studies would have likely attenuated any more extreme differences in the haematologic toxicity between the two regimens, if present.
The observed response rate was similar in this study compared with our prior trial. This finding is consistent with results from a study in paediatric rhabdomyosarcoma in which response rates were similar between a protracted (5 days per week × 2 weeks) and a shorter (5 days per week × 1 week) irinotecan schedule (Mascarenhas et al, 2010). As resource limitations precluded performance of tumour dosimetry in this study or in our prior study, we are not able to determine whether tumour dose varied by irinotecan regimen or between the two 131I-MIBG dose levels in this study. Our findings may indicate that exposure to irinotecan only during the first few days after 131I-MIBG administration, when radiation levels are highest, is sufficient. With this regimen, the total irinotecan dose per course is 250 mg m−2 compared with 200 mg m−2 in our prior study. Therefore, it is also possible that the use of higher doses of irinotecan in this study may compensate for a decrease in the number of days of overlap with radiation. Interestingly, the BM response rate was higher on the current study (33% vs 8% on the prior study). It is not clear whether this finding is because of higher irinotecan dose intensity or because of small numbers of patients in these two early-phase trials. As the overall response rate is similar to that reported in trials with single-agent 131I-MIBG (Wilson et al, 2014), a final possibility is that the irinotecan is not contributing to the antitumour activity of this regimen and therefore the schedule of administration is not relevant.
The UGT1A1 genotype correlates with risk of neutropenia in adult studies of irinotecan (Hoskins et al, 2007; Deeken et al, 2008). A meta-analysis of adults treated with irinotecan also demonstrated a correlation between the UGT1A1*28 allele and diarrhoea, although only at doses ⩾125 mg m−2 (Hu et al, 2010). A prior paediatric study investigated this issue in patients receiving irinotecan on protracted schedules and observed no correlation between the UGT1A1 genotype and haematologic or gastrointestinal toxicity (Stewart et al, 2007). Our previous trial of 131I-MIBG with vincristine and irinotecan also included a pharmacogenomics aim and likewise did not observe a difference in the rates of diarrhoea on the basis of the UGT1A1 genotype (DuBois et al, 2012). In the current study, patients homozygous for the UGT1A1*28 allele had twice the rate of grade 4 thrombocytopenia compared with other patients, although the difference was not statistically significant. Larger studies will be needed to assess this issue more definitively.
In conclusion, this regimen of 131I-MIBG with vincristine and shorter course, higher-dose irinotecan appeared to be more tolerable compared with our prior regimen using protracted, lower-dose irinotecan. The antitumour activity was similar to our prior regimen. Whether different 131I-MIBG administration schedules, such as double infusions targeted to specific whole-body radiation doses (Gaze et al, 2005; Matthay et al, 2009), might improve the response rate and further improve the tolerability of this regimen will require additional study. As the role of 131I-MIBG expands in the treatment of advanced neuroblastoma, it will be important to determine whether the addition of systemic radiation sensitisers improves the activity of 131I-MIBG. Towards that end, we are conducting an ongoing randomised phase II trial in which patients receive 131I-MIBG monotherapy, 131I-MIBG with vorinostat or 131I-MIBG with vincristine and irinotecan (NCT02035137). The shorter course, higher-dose irinotecan regimen reported here has been selected to move forward for further study in that ongoing trial.
Change history
17 February 2015
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, Gongora R, Manil L (1995) A new 123I-MIBG whole body scan scoring method—application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer 31A: 256–261.
Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, Maris JM, Kretschmar C, Cohn SL (2011) Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group Study. J Clin Oncol 29: 208–213.
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26: 404–413.
Deeken JF, Slack R, Marshall JL (2008) Irinotecan and uridine diphosphate glucuronosyltransferase 1A1 pharmacogenetics: to test or not to test, that is the question. Cancer 113: 1502–1510.
DuBois SG, Chesler L, Groshen S, Hawkins R, Goodarzian F, Shimada H, Yanik G, Tagen M, Stewart C, Mosse YP, Maris JM, Tsao-Wei D, Marachelian A, Villablanca JG, Matthay KK (2012) Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: a new approaches to neuroblastoma therapy trial. Clin Cancer Res 18: 2679–2686.
DuBois SG, Matthay KK (2008) Radiolabeled metaiodobenzylguanidine for the treatment of neuroblastoma. Nucl Med Biol 35 (Suppl 1): S35–S48.
DuBois SG, Messina J, Maris JM, Huberty J, Glidden DV, Veatch J, Charron M, Hawkins R, Matthay KK (2004) Hematologic toxicity of high-dose iodine-131-metaiodobenzylguanidine therapy for advanced neuroblastoma. J Clin Oncol 22: 2452–2460.
Gains JE, Walker C, Sullivan TM, Waddington WA, Fersht NL, Sullivan KP, Armstrong E, D’souza DP, Aldridge MD, Bomanji JB, Gaze MN (2014) Radiation exposure to comforters and carers during paediatric molecular radiotherapy. Pediatr Blood Cancer.
Gaze MN, Chang YC, Flux GD, Mairs RJ, Saran FH, Meller ST (2005) Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm 20: 195–199.
Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, Mcleod HL (2007) UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99: 1290–1295.
Hu ZY, Yu Q, Zhao YS (2010) Dose-dependent association between UGT1A1*28 polymorphism and irinotecan-induced diarrhoea: a meta-analysis. Eur J Cancer 46: 1856–1865.
Mascarenhas L, Lyden ER, Breitfeld PP, Walterhouse DO, Donaldson SS, Paidas CN, Parham DM, Anderson JR, Meyer WH, Hawkins DS (2010) Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol 28: 4658–4663.
Matthay KK, Panina C, Huberty J, Price D, Glidden DV, Tang HR, Hawkins RA, Veatch J, Hasegawa B (2001) Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med 42: 1713–1721.
Matthay KK, Quach A, Huberty J, Franc B, Hawkins R, Jackson H, Groshen S, Shusterman S, Yanik G, Veatch J, Brophy P, Villablanca JG, Maris JM (2009) 131I-Metaiodobenzylguanidine (131I-MIBG) double infusion with autologous stem cell rescue for neuroblastoma: a new approaches to neuroblastoma therapy (NANT) phase I study. J Clin Oncol 27: 1020–1025.
Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, Veatch J, Goldsby R, Brophy P, Kersun LS, Hawkins RA, Maris JM (2007) Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol 25: 1054–1060.
McCluskey AG, Boyd M, Ross SC, Cosimo E, Clark AM, Angerson WJ, Gaze MN, Mairs RJ (2005) [131I]meta-iodobenzylguanidine and topotecan combination treatment of tumors expressing the noradrenaline transporter. Clin Cancer Res 11: 7929–7937.
O’Leary J, Muggia FM (1998) Camptothecins: a review of their development and schedules of administration. Eur J Cancer 34: 1500–1508.
Pappo AS, Lyden E, Breitfeld P, Donaldson SS, Wiener E, Parham D, Crews KR, Houghton P, Meyer WH (2007) Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J Clin Oncol 25: 362–369.
Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, Shaw PJ, Cohn SL, Matthay KK (2011) Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children’s Oncology Group study. J Clin Oncol 29: 4351–4357.
Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC (2008) Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol 26: 190–195.
Stewart CF, Panetta JC, O’shaughnessy MA, Throm SL, Fraga CH, Owens T, Liu T, Billups C, Rodriguez-Galindo C, Gajjar A, Furman WL, Mcgregor LM (2007) UGT1A1 promoter genotype correlates with SN-38 pharmacokinetics, but not severe toxicity in patients receiving low-dose irinotecan. J Clin Oncol 25: 2594–2600.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216.
Treuner J, Feine U, Niethammer D, Muller-Schaumburg W, Meinke J, Eibach E, Dopfer R, Klingebiel T, Grumbach S (1984) Scintigraphic imaging of neuroblastoma with [131-I]iodobenzylguanidine. Lancet 1: 333–334.
Wagner LM, Crews KR, Iacono LC, Houghton PJ, Fuller CE, Mccarville MB, Goldsby RE, Albritton K, Stewart CF, Santana VM (2004) Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin Cancer Res 10: 840–848.
Wagner LM, Crews KR, Stewart CF, Rodriguez-Galindo C, Mcnall-Knapp RY, Albritton K, Pappo AS, Furman WL (2008) Reducing irinotecan-associated diarrhea in children. Pediatr Blood Cancer 50: 201–207.
Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN (2014) A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer 50: 801–815.
Acknowledgements
We gratefully acknowledge participating patients and families as well as research nurses and research assistants. This study was supported by the Thrasher Pediatric Research Fund (SGD), Campini Foundation (SGD and KKM), Dougherty Family Foundation (KKM), NIH P01 CA81403 (KKM) and Alex’s Lemonade Stand Foundation (SGD and KKM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Prior Presentations: Presented in part at the 2012 Advances in Neuroblastoma Research meeting and at the 2014 American Society of Clinical Oncology Annual Meeting.
Disclaimer
The funding sources did not have a role in study design, conduct, data analysis or interpretation.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
DuBois, S., Allen, S., Bent, M. et al. Phase I/II study of 131I-MIBG with vincristine and 5 days of irinotecan for advanced neuroblastoma. Br J Cancer 112, 644–649 (2015). https://doi.org/10.1038/bjc.2015.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.12
Keywords
This article is cited by
-
The efficacy and safety of Iodine-131-metaiodobenzylguanidine therapy in patients with neuroblastoma: a meta-analysis
BMC Cancer (2022)
-
Phase I/II clinical trial of high-dose [131I] meta-iodobenzylguanidine therapy for high-risk neuroblastoma preceding single myeloablative chemotherapy and haematopoietic stem cell transplantation
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
EANM Dosimetry Committee series on standard operational procedures for internal dosimetry for 131I mIBG treatment of neuroendocrine tumours
EJNMMI Physics (2020)
-
18F-meta-fluorobenzylguanidine (18F-mFBG) to monitor changes in norepinephrine transporter expression in response to therapeutic intervention in neuroblastoma models
Scientific Reports (2020)
-
High-dose 131I-metaiodobenzylguanidine therapy in patients with high-risk neuroblastoma in Japan
Annals of Nuclear Medicine (2020)