Abstract
Background:
The way in which patients receive bad news in a consultation can have a profound effect in terms of anxiety, depression and subsequent adjustment. Despite investment in well-researched communication skills training and availability of decision-making aids, communication problems in oncology continue to be encountered.
Methods:
We conducted a mixed-methods study in a large UK Cancer Centre to develop a novel consultation aid that could be used jointly by patients and doctors. Consultations were audio-recorded and both the doctors and the patients were interviewed. We used conversation analysis to analyse the consultation encounter and interpretative phenomenological analysis to analyse the interviews. Key themes were generated to inform the design of the aid.
Results:
A total of 16 doctors were recruited into the study along with 77 patients. Detailed analysis from 36 consultations identified key themes (including preparation, information exchange, question-asking and decision making), which were subsequently addressed in the design of the paper-based aid.
Conclusions:
Using detailed analysis and observation of oncology consultations, we have designed a novel consultation aid that can be used jointly by doctors and patients. It is not tumour-site specific and can potentially be utilised by new and follow-up consultations.
Similar content being viewed by others
Main
It is well recognised that the way in which patients receive bad news in a consultation can have a profound effect in terms of anxiety and depression and subsequent adjustment (Fallowfield et al, 2002; Schofield et al, 2003). This is reflected in both the NHS Cancer Plan for England and Wales and the National Institute for Clinical Excellence (NICE) Improving Outcomes Guidance in Cancer Services (Department of Health, 2000; NICE, 2004). Both documents emphasise good communication between health professionals and patients as being integral to delivering high-quality care and empowering people to be involved in decisions about their own care. The NICE document states that ‘individual preferences for different levels of involvement in decisions must be respected’. Consequently, there has been significant investment in well-researched communication skills training for doctors (Fellows et al, 2004) and there is a national communication skills training programme run through the National Cancer Action Team (National Cancer Action Team website; National Cancer Action Team Connected; Advanced Communication Skills Training, http://ncat.nhs.uk/our-work/improvement/connected-advanced-communication-skills-training#).
Other tools available in oncology to enhance patient participation in consultations include:
-
1
Information-based interventions—for example, using patient question prompt sheets, giving patients audiotapes of the consultation or written reports (Butow et al, 1994; Brown et al, 2001; Bruera et al, 2003; Clayton et al, 2007).
-
2
Coaching/skill-building approaches—for example, educating patients with skilled trainers before their oncology consultation to ‘rehearse’ the consultation (Stacey et al, 2012).
-
3
Community-based approaches—for example, workshops where patients are told about the consultation process and what to expect (Towle et al, 2003).
-
4
Decision-making aids—for example, written algorithms of the options available in a specific disease area (Silvia et al, 2008).
Although all these options have some merit, none are ideal. Information-based interventions cover a broad group of communication aids with examples including promotion of question-listing before consultation (Kinnersley et al, 2007), audio-recording consultations and note-taking services (Pitkethly et al, 2008). The main aims of these interventions are to increase question-asking and information recall following the consultation. Numerous such aids and approaches are available in oncology and results are mixed as described in the Cochrane reviews cited.
Both coaching- and community-based approaches are excellent in preparing patients for what to expect at their consultation and this can ensure that patients have the skills and confidence to participate fully in their consultation. However, as this is done in isolation from the doctors, it does not help doctors structure their approach to the consultation.
Decision aids are print or multimedia materials that present focused information about treatment options and their outcomes. The Cochrane Collaboration systematic reviews of interventions involving decision aids for patients making health treatment or screening decisions (Stacey et al, 2011) concluded that these aids increased patients’ knowledge, reduced decisions conflict, reduced the number of patients being passive in decision making and reduced the number of patients being undecided about their treatment. However, there was no demonstrable effect on patient satisfaction or anxiety and no clear understanding of the impact on the patient–doctor encounter (e.g., consultation length).
An increasingly important factor in effective communication in oncology is the recording of patient-related outcomes (PROs) such as quality of life. An interesting randomised trial enabled PROs to be fed back to managing oncologists in ‘real-time’ (Velikova et al, 2004; Takeuchi et al, 2011). This approach did seem to improve communication around symptoms but not necessarily around functioning problems and they concluded that more clinician-focused strategies were required.
Many of the types of aids referred to above are often disease specific; hence, their application is limited. Moreover, these aids and training approaches have tended to play down the dynamic nature of doctor–patient communication and in particular the role of the doctor’s consulting behaviour in shaping patient involvement. Some excellent clinical guidelines for medical practitioners do exist (Baile et al, 2000). Although they do highlight the importance of assessing the patients’ perception of their situation and seek to engage them in the consultation, they target the doctors’ behaviour and so lack transparency for the patient in relation to their involvement in the consultation process. Compounding this, in a study by our group, doctors, particularly trainees, have reported their lack of confidence in structuring consultations. They frequently experience a ‘mis-match’ between eliciting the patient’s agenda and the disclosure of necessary information (e.g., prognosis or scan result) at the time of consultation (Furber, 2010). From our perspective, both parties’ communication behaviours need to be examined in concert with one another to identify how that dynamic influences and shapes the consultation process (Heritage and Maynard, 2006).
On the basis of the literature, we believe that there is an unmet need with the consultation aids currently available as none are specifically designed to be used jointly by the patient and the doctor with the key intention being to influence the consultation behaviour of both parties and make transparent the patients’ prior understanding of their illness and need for further information. Therefore, the aim of this study was to develop a consultation aid that could be used jointly by doctors and patients and undertake preliminary evaluation of the aid with focus groups. This article reports some central findings from the study and discusses how this led to the development of a consultation aid and how this aid differs from others in the literature.
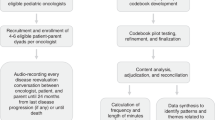
This was a mixed-methods study using questionnaires and semi-structured interviews with doctors and with patients attending oncology consultations. Ethical approval for the study was obtained from Nottingham Research Ethics Committee 2 (reference number 09/H0408/34) and all participating subjects (doctors and patients) gave written informed consent, which included the use of anonymised quotes.
Section 1 methods
Setting
The study was carried out in a large UK Cancer Centre that provides all non-surgical oncology specialist treatment options.
Recruitment process
To provide as much data as possible to inform the design of the consultation aid, a maximum variability sample for both the patients and the doctors was required. All oncology consultants and specialist registrars (SpRs) working in the oncology department (both medical and clinical oncologists) during the recruitment period were invited to participate in the study. This was done initially by means of a participant information letter followed by a face-to-face discussion with a member of the research team. We then liaised with the participating consultants’ clinic co-ordinators weekly in order to identify eligible patients. We aimed to recruit up to two patients from consecutive clinics (numbers were limited per clinic to ensure we could organise subsequent interviews in a timely manner). We purposefully sampled to ensure a wide range of patients with a variety of tumour types and on different treatment pathways. We recruited not only patients who were new referrals to the cancer centre but also those attending for chemotherapy, radiotherapy or follow-up and undergoing active surveillance. They had to know their cancer diagnosis, be over 18 years of age and be willing to participate in the study. LF and SB provided an opportunity for doctors and patients to seek further clarification about the study and obtained written informed consent from those willing to participate.
Study procedures
Participants consented to having their consultations audio-recorded. The researchers (LF or SB) placed a small digital audio recorder on the desk in the consulting room. When the doctor indicated they were ready to see the patient, the researcher pressed record and left the consulting room.
Immediately following the consultation the patients completed the Patient Satisfaction Questionnaire PSQ-MD (Loblaw et al, 1999, 2004) to record their satisfaction with that consultation. The questionnaire was scored (see Analysis section) and those patients with high, moderate and low satisfaction scores were then invited to undergo a semi-structured interview. This was conducted in the hospital or in the patient’s home within 1–4 days following the consultation. Doctors were also interviewed as soon as possible after the consultation and only ever had one trial participant per clinic. The interviews were conducted by LF and SB, audio-recorded and transcribed verbatim.
The content of the interview guide for doctors and patients was similar. Each had a set of open-ended questions directed to exploring, for example, how participants felt before, during and following the consultation; how they each experienced the consultation in terms of interactions and communication; how information was shared and received; and their satisfaction with the consultation.
Analysis plan
Identification of patients for interviewing using the PSQ-MD
The PSQ-MD questionnaire contains 24 items and is anchored by a 4-point Likert-type response scale (strongly agree, agree, disagree and strongly disagree) as well as by the response ‘does not apply’. We scored these positively such that four equated with strongly agree. As some elements of the PSQ-MD are negatively worded, and others positively worded, we used reverse scoring as necessary. ‘Does not apply’ was recorded as missing data. For each participant completing the PSQ-MD questionnaire, we recorded their score as a mean with four equating to very satisfied—this was in line with the methodology applied by the developers of the scale. As a pre-requisite for the methodology for this study we carried out a piece of work where we gave the PSQ-MD questionnaire to 205 patients attending our Centre to obtain scores from a suitable reference population. In that study, the return rate of questionnaires was 83.9%, and of these over 90% were successfully completed. Results indicated a mean satisfaction score of 3.51 SD 0.40 with a range of 1.95–4.0. The satisfaction scores were non-normally distributed and it was not possible to transform the data to a normal distribution. Consequently, in the current study, we proposed to select all patients below the 25th percentile (approximately) and all patients above the 75th percentile (approximately) and a sample of 10 patients from the 37.5–62.5 percentile, thus representing patients with high, moderate and low satisfaction. Patients were recruited consecutively. In addition, as requested by the Patient and Carer Study Group (PCSG), we agreed to purposefully sample a small number of patients whose score did not fall within one of these groups, however, a significant event happened during the consultation (for example, test results were unavailable).
In line with other qualitative studies a formal power calculation for recruitment numbers was not carried out (Hancock et al, 2009), and we aimed to recruit 30–40 patients for interview. To maximise the number of clinics we could recruit from, we planned to recruit as many doctors as possible.
Conversation analysis
We applied conversation analysis (CA) (Collins et al, 2005; Heritage and Maynard, 2006) to the audio-recordings of the consultations, which allowed us to undertake a meticulous examination of each of the encounters. Characteristics of speech exchange were detailed with the location of utterance types, including patient-initiated questions, topic transition statements, pauses, pace and intonation. These characteristics, among others, provided important insight into the structure and process of the interaction and how that process was influenced by the communication behaviours of the doctor and the patient. The first level of analysis was conducted by GM. Subsequent analyses were carried out by GM, AT and LF. Any disagreements regarding the interpretation of the data were resolved through discussion using findings from the interview data as well as by revisiting the original consultation data.
Intrepretative phenomenology analysis
We carried out intrepretative phenomenology analysis (IPA) on the interview data from doctors and patients. We analysed the expectations and experiences of both patients and doctors individually before looking for shared themes across the data. Intrepretative phenomenology analysis, an approach outlined by Smith et al (2009), is used to establish an in-depth, experientially focused analysis and is useful for exposing complexity within and between participant accounts. Initially, each interview was subjected to analysis by LF and SB independently. The researchers then compared their coded data and how they each interpreted the experiences described by each participant. Patterns of meanings were identified and recorded, and subsequently tentative comparisons were made across cases. Maps were created to look at interrelationships, connections and patterns from the data to identify key themes and subthemes.
Section 1 results
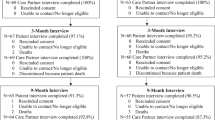
Patients and doctors were recruited over a 12-month period. A total of 16 doctors were recruited (7 out of 9 potential consultants and 9 out of 12 registrars) of whom 6 were male and 10 were female. The main reason for doctors not wishing to take part was lack of time to guarantee availability for interviews. Four doctors did not take part in any interview and the median number of interviews per doctor was 3 (range 1–5). A total of 182 patients were approached and of them 77 agreed to take part in the study (see Figure 1). Demographic data of the participants are shown in Table 1. We reached saturation of emerging themes after analysing the CA and IPA data from 36 patients. This included four patients who were recruited because a significant event happened in the consultation (e.g., pivotal clinical data missing), although the satisfaction score did not fall within one of the targeted percentile ranges.
PSQ-MD analysis
It was assumed that data were missing at random and as such could be excluded (subsequent analysis reviewing patterns of missing data confirmed the accuracy of this assumption). The scores per percentile and the number of patients recruited per group are shown in Figure 2. Although we had previously tested the PSQ-MD questionnaire, it did not perform well in identifying patients with high and low satisfaction. Discordant results were found between the score from the questionnaire and the information given at the semi-structured interviews. For example, when answering the question ‘The doctor did not take my problems very seriously’ only 1 out of 8 patients who reported dissatisfaction actually described being dissatisfied during their interview. Some patients scored a low satisfaction score but described the consultation in positive terms during the interview. For all quotes, participants are identified by their status of patient/doctor, their gender and participant number.
Key themes
In total, we identified six key themes (Table 2).
Preparation for the consultation
Preparation for the consultation emerged as a critical factor in shaping doctors’ and patients’ perception of the consultation. From the patients’ perspectives, there was consensus that, irrespective of how good their consultation experience was, a diagnosis of cancer brought distress and this could not be avoided. However, having information about what was likely to happen at an oncology consultation in advance could at least minimise the anxiety of this encounter.
All of the doctors read annotations and looked at results before seeing the patient. Only one doctor, DM2, described how he felt it was inappropriate to ever read notes or look at the computer screen in front of the patient. DM2 thought it helped the patient know that they were the main focus of his attention. Patients on the other hand did not mind if the doctor had to refer to their notes for clarification during the consultation but some considered it disrespectful to sit reading notes in front of them.
‘It is very clear when you walk into a room and the doctor hasn’t a clue about you. They are trying to read your notes while you are talking to them and they can’t do the two things at once.’ (PM62)
PF46 described how doctors frequently read her notes in front of her making her feel like a ‘set of notes, ‘dehumanised’ and ‘lost within a system’. In contrast, PM62 described how satisfied he was with a consultation because, even though he had never met the doctor before, she had taken the trouble to review his notes and so already knew something about him.
Patients also reported that it was important that medical notes be prepared sufficiently and that medical reports or referral letters be present and correct in the patient’s notes. In at least 5 out of the 36 consultations this was not the case, which had a negative impact on the doctor’s and patient’s consultation experience.
‘I felt cross and I felt frustrated because this was a vital piece of information. Why didn’t somebody phone up, you know worst case scenario and say look I’m really sorry there’s a piece of information missing that we need for this consultation, so would it be possible to postpone the consultation.’ (PM34)
‘… the case notes were just not properly prepared. I just do not know why that appointment wasn’t cancelled. We derived no benefit whatsoever from that consultation.’ (PF27)
In each of these five cases, the doctors expressed frustration because without the necessary information they could not produce a definitive management plan. Although patients wanted clinical decisions to be made in a timely and efficient manner, coming to the hospital was an effort for many patients. With this in mind, three of the patients described how they would rather be given the opportunity to postpone their consultation until the information was available; it was considered a waste of their time and of hospital resources. Forward planning and honesty was regarded as the best policy by these patients on such occasions.
Agenda mismatch
Frequently during the consultation there was a clear ‘agenda mismatch’. That is to say, the information needs and priorities of the patient were often misaligned with those of the doctor. The reason for this appeared to be multifactorial but often lay at the heart of the communication process. This became apparent when comparisons were made between the doctors’ and the patients’ perspectives following the consultation. For example, following one consultation the doctor thought that her consultation with a patient had been very straightforward and pleasant, but in contrast the patient was disappointed because the doctor had not acknowledged with her the significance of completing a long and difficult course of treatment for breast cancer and had concerns for her future.
‘I think she was obviously wondering what the echo report was like but I don’t think there is anything else she was worried about. She came in relaxed and just seemed calm. It was an enjoyable experience.’ (DF8)
‘It would it have been nice if she’d spoken to me a little bit about my coming to the end of my treatment; because it’s a big thing to have gone through two years almost of treatment and to be coming to the end of the major part of it.’ (PF46)
PF46 explained that once she stopped treatment she would lose the ‘safety net’ of the hospital, but did not articulate this concern to her doctor. This reflects a typical behaviour we observed, which is that patients generally chose not to question the doctor or seek further information to meet their own needs.
At times, patients were given minimal opportunity to share their concerns. In particular, some SpRs feared that eliciting a patient’s concern would lengthen the consultation or produce issues they did not have the skills to deal with:
‘I usually wait for the patient to open up to me because probing can be quite difficult. I also think sometimes that there’s not enough time in the consultation to probe everyone. I think I’d only start probing someone for information if I was concerned that they might be depressed or giving off clues that they might be depressed.’ (DF8)
When asked to describe what they thought the patient’s main needs were during the consultation eight doctors were only able to guess. In contrast, most patients valued the opportunity to raise their concerns in the consultation and 13 patients described this aspect as being very important.
Improving information exchange
Timing of information delivery
In order to improve the exchange of information, doctors described needing to give information to patients clearly, and in stages. Four doctors believed that patients needed to know that there would be more opportunity in the forthcoming weeks to learn more about their illness and that on each occasion patients needed time to allow the information to sink in. As one doctor described it:
‘Often it takes more than one consultation before they understand all these details. It is often very difficult to come in and hear about a very complicated treatment…There are lots of stops for them to get more information…so on the whole we tend to give them little bits of information at a time rather than a whole load in one go.’ (DM6)
Patients tended to review the speed in which they received information differently. Receiving information about a diagnosis of cancer warranted a speedy disclosure so that patients were not left in a state of limbo. Throughout the patients’ care pathway six patients described not wanting to know all of the facts too soon. If too much information was given in one consultation, patients found it difficult to absorb and tended to feel more anxious.
Patients who were more likely to feel satisfied with their consultation experience received information when they were ready to hear it and were made to feel involved in their care.
Encouraging question-asking
It was not uncommon for patients to prepare a list of questions but this did not mean they referred to it. From the CA, we discovered that levels of patient-initiated questions within the consultation were very low. For most patients, impression management, that is, the patient’s desire to influence the doctor’s impression of them (Goffman, 1959), seemed to be paramount in shaping levels of question-asking. For example, four patients were concerned about asking questions because they did not want to appear to be challenging the doctor’s competence, or as one patient put it ‘rocking the boat’. Other patients were concerned that asking questions implicated their own lack of intellect. Finally, five patients reported that they were acutely aware of taking up the doctor’s time and were reticent to ask questions so as not to lengthen the consultation and contribute to further delays in the clinic.
Ten patients articulated the importance and value of viewing their scan results on the computer as this made them feel included in their care. Moreover, reviewing digital images also enabled patients to ask their questions and at times aided understanding. This finding was verified in our analysis of the consultation data, which revealed that, almost invariably, patient-initiated questions followed when doctors invoked scan or X-ray results in the consultation process (Murtagh et al, 2013).
‘I understood what he was talking about and he showed me the scan. He asked if I wanted to see it and I said yes. I thought it was pretty good the way he explained it...I then understood why I couldn’t eat very fast or why it was coming back.’ (PM19)
Decision making
It is very challenging for doctors to know how involved patients want to be in decision making, particularly when they are meeting them for a first consultation and at a time when emotions are high. Areas for caution were highlighted. Even if patients wanted to be involved in decision making they were mindful that they did not have the knowledge to make decisions about their care on their own and turned to the doctor for their expertise and guidance. As such they expected the doctor to advise the best course of action and often found it very difficult if options were given with no guidance. There was a misunderstanding among some doctors that patient centredness involved leaving the patient to make all the decisions. This is exemplified in the following extract:
‘…they said you can have chemo if you want it, but it’s my decision. I found it very hard to make a decision like that because they said no decision’s the right one or wrong one….. I said what would you do if you were me and he said I can’t tell you that, you’ve got to make the decision yourself. I found that very hard but obviously that is the way things are…’ (PF13)
In some cases, a lasting impression was made if a patient felt the doctor had communicated insensitively and/or forced a patient to make a decision they felt ill-equipped to make. One patient refused to see one doctor because she believed he had been unprofessional:
‘We went in and he said ‘Are you going to have chemo or radiotherapy? Well, when you’ve just gone into a room and they say are you going to have this or that, I mean, as ignorant bystanders we don’t know the ins-and-outs of things and well, we don’t know; perhaps we’ll have to think about it. ‘Well, you’ve got to make your mind up because I’ve got to fill this form in, and if you don’t have it done you’ll be gone in 18 months.’ (PF24)
Prognosis
We also discovered that information about prognosis was poorly handled, and on some occasions doctors and patients felt reluctant to raise the topic. For patients, it was important that a consistent message was conveyed throughout their care. Four patients reported being given inconsistent information and felt dissatisfied and somewhat untrusting of their doctors. Words used to convey a message from the outset stayed with a patient throughout their care. For example,
‘I always remember something that was said to me at my first interview. This is a very aggressive carcinoma and difficult to control. We’ll teach it a lesson and give it a good thrashing first time round but don’t lose sight of the fact that it’s an aggressive one to treat and difficult to cure.’ (PM67)
Additionally, when the issue of prognosis was raised in the consultation it was important that the information was delivered sensitively and timely—preferably not just before the end of the consultation. Many patients appreciated that it must be a difficult task to break bad news and felt sympathetic towards the doctor for having to do this part of their job. Patients were appreciative if the doctor treated them as an individual and were sensitive to their needs when disclosing information and offered them some hope that all would be done to help and support them. PM62 reported that giving the patient time was critical when sensitive information is exchanged—‘this is your time and it doesn’t matter how long it takes’. Patients also recognised the importance of subtle communication behaviours (e.g., maintaining eye contact and expressions of empathy) as positively influencing the delivery of prognostic information.
Scetion 2 methods
To develop the consultation aid, we identified specific communication practices using CA and key themes emergent from the interview data using IPA. The researchers consulted regularly throughout the analysis period and comparisons were made between emerging CA and IPA themes until we were confident of theme saturation. This provided us with insight into both the unique and general patterns of consultation behaviour for both doctors and patients. The PCSG was consulted monthly through the analysis period and provided another layer of analysis, helping us develop the design of the aid. The final aid was approved by the research team and PCSG before taking to doctor and patient focus groups.
Focus group work
We prepared to conduct four focus groups with six to eight participants with a similar background in each group. For the patient focus groups, clinic co-ordinators and Clinical Research Nurses were asked to identify patients willing to participate—none had taken part previously in the study. Patients were either undergoing treatment or had recently completed treatment and were being followed up by their oncologists. Each doctor working within the oncology department was invited to participate in a focus group. We proposed to hold one focus group with consultants and one focus group with registrars. Having received a letter inviting them to participate in the focus group, each participant provided written consent.
All focus groups were audio-recorded; the patient groups were faciliated by LF and the doctor focus groups by SB. They were held in a meeting room in the oncology department and it was anticipated that each focus group would last for ∼60 min. A simple topic guide was used to faciliate the focus group discussions. We wanted to know what the participants felt about the design and content of the consultation aid prototype and how they perceived the consultation aid might be used in practice. A group discussion was encouraged. The data provided were used to finalise the design and produce a prototype of the Consultation aid for future validation and acceptability testing.
Section 2 results
Four consultants attended their focus group, with two additional consultants meeting with the researcher at a later time. Seven registrars attended their focus group. Three doctors had not taken part in the study previously. Three patient focus groups were held with five, four and two patients in each. The main reason for patients not attending the group as planned was due to a change in clinical situation.
Design of the aid
One of the central findings from our data is that patients’ needs and expectations are significantly influenced by their prior experience and these can change over a period of time. We recognised that any aid would need to effectively accommodate this. It seemed inappropriate to develop an aid for patients only, when it was clear from our data that doctors too needed to recognise how they could improve their consultation behaviour. On the basis of the literature and our findings, we were keen to develop an aid that could be used jointly by the patient and the doctor. We therefore designed a paper-based consultation aid. It consists of two booklets (one for the doctor and one for the patient) and leaflets to be completed by the patient before consultations (one for new consultations and one for follow-up). It was anticipated that the patients would complete the leaflet before seeing the doctor and this would go on the front of the case notes. The doctor would review this as part of the preparation for the structure of the consultation. Although we did consider a web-based aid, the paper style was cheap and did not rely on patients’ computer skills and hence we hoped that it would be acceptable to a wider audience.
In the doctor booklet, we described the aid and how we envisaged patients would use it. We also gave some reminders on how to improve the consultation experience—for example, being aware of interpersonal skills and assessing patients’ needs and expectations. Throughout the aid we used patients’ quotes from our study as these were a very powerful way to illustrate key issues. It was anticipated that doctors would not use the booklet with each consultation but rather have it as an aid memoir to consult as necessary.
In the patients’ booklet we explained the different types of oncology consultations in some detail, including who to bring, what will happen and who they will see. We also talked about timings of the clinic and consultation so that patients had an idea of how much time they had dedicated to them. We explained about question-asking as well as decision making to provide patients with clear insight into ways in which they could structure their involvement and get the most from their consultation.
The patient leaflets asked patients to consider how much information they wanted about their prognosis and to write down any particular questions. For the follow-up consultations patients were asked to consider how they wanted to be involved in the consultation.
Focus group feedback
There was overwhelming support from the patients. They felt the layout and content of the consultation aid was just right. They anticipated that relatives of patients in particular would find the booklet helpful. Even some of the more ‘experienced’ patients who had been attending the oncology department for a long time learnt new things (for example, being able to see their scan results).
Interestingly, there was also significant support from the more senior doctors. They felt that the design and content of the consultation aid was appropriate and would be useful in practice. They felt that they would have greater insight into the needs of their patient and the patient’s current level of understanding. They also hoped that the patient would become more focused and would be empowered to take greater sense of control and ownership about what was happening to them if they so wished. In contrast, the junior doctors were concerned about the aid lengthening the time of the consultations. It was very clear from their discussion that they were focused on delivering the information they perceived they had to with patient needs taking second place. There was also concern raised that specifically bringing up the issue of prognosis at an early stage could heighten anxiety and depression. Finally, doctors wanted reassurance that the aid could be introduced into the current patient pathways and not introduce delays at any stage.
Discussion
We have developed a consultation aid that can be used jointly by doctors and patients in both new and follow-up consultations for patients in oncology. It is not tumour-site specific and can be used in a longitudinal manner by patients throughout their cancer journey. As such, to the best of our knowledge it is novel compared with other types of aids used in oncology consultations.
A limitation of our study is that the PSQ-MD did not perform as well as we had predicted from our previous experience. We found that there were circumstances when the satisfaction score predicted that a patient would be dissatisfied with their consultation, and yet at interview positive comments were made, and vice versa. Of course, this may be because the recollection of the consultation may change once a patient has time to reflect on its content. By selecting patients with a range of satisfaction scores we do not think that this observation would have biased the emergent themes used for the design of the aid. We recognise how difficult it is to measure satisfaction in the clinical setting. Although in their second paper Loblaw et al identified two subscales of the PSQ-MD (physician disengagement and perceived support), our interview data did not show any correlation with either of these subscales.
We purposefully wanted to develop an aid that had the potential to be used by many patients, and therefore we recruited patients with different tumour types on various treatment pathways to try and capture as many important themes as possible. A caveat is that, because of the small numbers recruited into each group, we are unable to assess whether the performance of the PSQ-MD varied between clinical groups. Another limitation of our study is that the take-up rate for participation by patients was low. We are therefore aware that there may be ‘missed’ important themes within this population.
We identified six main emergent themes, all of which have previously been reported to some extent when patient satisfaction has been studied. We were surprised with the number of doctors who misunderstood the term patient-centredness and believed this to be passing all information to the patient and remaining distant from any clinical management making. We were also surprised with the low level of question-asking that was seen in our patient population compared with other studies (Butow et al, 1994; Murtagh et al, 2013). The finding that actually showing the digital scan images to patients promoted question-asking was to our knowledge a novel strategy for promoting information exchange. Despite all of our participating consultants attending an advanced communication skills training course, negative ‘off the cuff’ remarks were still evident. The long-standing impact on the patient experience of these unintentional but ultimately insensitive comments was clear.
Rodin carried out a systematic review of practice guidelines, systematic reviews and randomised trials specifically in cancer (Rodin et al, 2009). He concluded that some of the evidence reviewed supported the view that there was a psychological benefit to patients (mainly women and those with lower levels of anxiety) when discussions of life expectancy and prognosis are included in consultations. Non-specific physician characteristics, such as compassion and empathy, are important in improving patient satisfaction, and techniques to increase patient participation in decision making were associated with greater satisfaction, although they did not necessarily decrease distress. Overall, they concluded that an individualised approach was needed to meet patient communication preferences and styles. Our aid certainly has the potential of fulfilling this ambition.
Another personalised approach is ‘Decision coaching’: individualised, non-directive facilitation of patient preparation for shared decision making. It is usually provided by genetic counsellors, nurses, pharmacists, physicians, psychologists or health educators before the consultation. Part of a coaching approach may be to use an individualised patient decision aid. In their review, Stacey et al (2012) found that, compared with usual care, decision coaching improved knowledge. However, the improvement in knowledge was similar when coaching was compared with the use of a decision aid alone. It therefore brings into question the use of a coaching strategy with the necessary manpower and associated costs. Our aid requires limited resource for training and delivery and therefore is a much cheaper alternative.
Even with all these available interventions, very few are utilised in everyday practice. Some are so disease specific that their application is limited or alternatively they are too general for the needs of individual patients. Our findings support those of previous studies that report that some doctors are hesitant about using aids, particularly if they believe they will lengthen the consultation (Short et al, 2004). This concern may, however, be short sighted. Investing time in one consultation to deal with important issues raised by patients may shorten subsequent consultations (Brown et al, 2001).
Despite advances in communication skills training, it is clear that doctors are often uncertain or hesitant about what information to disclose to patients about their diagnosis and poor prognostic outlook and may avoid disclosing information if they are uncertain about what the patient may already know or want to know (Ptacek and McIntosh, 2009). Our findings demonstrated the need for a consultation aid that informs patients about the consultation process and how they might like to prepare for the consultation. Any consultation aid also needs to facilitate communication by minimising misunderstandings between doctor and patient, making apparent the patient’s current level of understanding about their diagnosis, desire for prognostic information and the patient’s desire to be involved in decision-making activities. This could reduce the rigidity of rituals and routines of consulting behaviour that doctors and patients engage in Kinnersley et al (2008).
In addition, unlike some of the current interventions, our aid actually reflects the dynamic of the doctor–patient interaction and how that shapes both parties’ conduct in their communication with one another. Patient behaviour cannot be seen in isolation from the doctor’s; to improve practice there is a requirement to analyse both parties in interaction with one another (Butow et al, 2004). The consultation aid we have designed certainly addresses this issue.
In conclusion, our study provides a rich insight into the experience of patients and doctors taking part in consultations in oncology. We have used CA to unpack some of the key component parts that shape typical patterns of the dynamic of the doctor–patient interaction. By combining observations from this detailed analysis as well as information from semi-structured interviews we have developed a new consultation aid. Feedback from focus groups set up to establish whether the design of the aid was acceptable and whether it should be used in practice has been informative. Patients were very positive; however, feedback from doctors was more mixed with concerns about the aid lengthening the consultation and heightening anxiety. All too often consultation aids are not embedded into clinical practice and therefore they become an underutilised resource. For this consultation aid to be rolled out into clinical practice it is essential that we answer the concerns of the doctors to make sure that they have confidence in its function. The aid requires full validation and therefore we propose to carry out an intervention study utilising the aid to ensure that it can be introduced into the patient pathway successfully and improve information exchange and patient experience.
Change history
04 March 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Baile WF, Buckman R, Lenzi R, Glober G, Beale EA, Kudelka AP (2000) SPIKES—A six-step protocol for delivering bad news: application to the patient with cancer. Oncologist 5: 302–311.
Belkora JK, Teng A, Volz S, Loth MK, Esserman LJ (2011) Expanding the reach of decision and communication aids in a breast care center: a quality improvement study. Patient Educ Counsel 83 (2): 234–239.
Brown R, Butow PN, Tattersall MHN (2001) Promoting patient participation and shortening the cancer consultation: a randomised trial. Br J Cancer 85 (9): 1273–1279.
Bruera E, Sweeney C, Willey J, Palmer JL, Tolley S, Rosales M, Ripamonti C (2003) Breast cancer patient perceptions of the helpfulness of a prompt sheet versus a general information sheet during outpatient consultations: a randomised controlled trial. J Pain Symptom Manage 25 (5): 412–419.
Butow PN, Devine R, Boyer M, Pendlebury S, Jackson M, Tattersall MHN (2004) Cancer consultation preparation package: changing patients but not physicians is not enough. J Clin Oncol 22 (21): 4401–4409.
Butow PN, Dunn SM, Tattersall MHN, Jones QJ (1994) Patient participation in the cancer consultation: Evaluation of a question prompt sheet. Ann Oncol 5: 199–204.
Clayton JM, Butow PN, Tattersall MH, Devine RJ, Simpson JM, Aggarwal G, Clark KJ, Currow DC, Elliott LM, Lacey J, Lee PG, Noel MA (2007) Randomized controlled trial of a prompt list to help advanced cancer patients and their caregivers to ask questions about prognosis and end-of-life care. J Clin Oncol 25 (6): 715–723.
Collins S, Drew P, Watt I, Entwistle V (2005) ‘Unilateral’ and ‘bilateral’ practitioner approaches in decision-making about treatment. Soc Sci Med 61: 2611–2627.
Department of Health (2000) The NHS Cancer Plan. Department of Health London.
Fallowfield L, Jenkins VA, Beveridge HA (2002) Truth may hurt but deceit hurts more: communication in palliative care. Palliat Med 16 (4): 297–303.
Fellows D, Wilkinson S, Moore P (2004) Communication skills training for health care professionals working with cancer patients, their families and/or carers. Cochrane Database Syst Rev 2: CD003751.
Furber L (2010) Investigating Interactions: How Do Doctors and Patients Experience the Disclosure of Significant Information in the Advanced Cancer Setting and How Do These Experiences Enhance Practice? [PhD]. University of Nottingham: Nottingham.
Goffman E (1959) The Presentation of Self in Everyday Life. Penguin Books: England.
Hancock B, Ockleford E, Windridge K (2009) An Introduction to Qualitative Research. NIHR RDS East Midlands/York & Humber. Report.
Heritage J, Maynard DW (2006) Communication in Medical Care: Interaction Between Primary Care Physicians and Patients. Cambridge University Press: Cambridge.
Kinnersley P, Edwards A, Hood K, Cadbury N, Ryan R, Prout H, Owen D, Macbeth F, Butow P, Butler C (2007) Interventions before consultations for helping patients address their information needs. Cochrane Database Syst Rev 3: CD004565.
Kinnersley P, Edwards A, Hood K, Ryan R, Prout H, Cadbury N, Macbeth F, Butow P, Butler C (2008) Interventions before consultations to help patients address their information needs by encouraging question asking; systematic review. Br Med J 337: a485.
Loblaw DA, Bezjak A, Bunston T (1999) Development and testing of a visit specific patient satisfaction questionnaire: The Princess Margaret Hospital satisfaction with doctor questionnaire. J Clin Oncol 17: 1931–1938.
Loblaw DA, Bezjak A, Singh PM, Gotowiec A, Joubert D, Mah K, Devins GM (2004) Psychometric refinement of an output visit specific satisfaction with doctor questionnaire. Psychooncology 13: 223–234.
Murtagh GM, Furber L, Thomas AL (2013) Patient initiated questions: how can doctors encourage them and improve the consultation process? A qualitative study. BMJ Open 3: e003112.
National Institute of Clinical Excellence (2004) Guidance on cancer services. Improving Supportive and Palliative Care for Adults with Cancer. NICE: London.
O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, Tait V, Tetroe J, Fiset V, Barry M, Jones J (2003) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2: CD001431.
Pitkethly M, Macgillivray S, Ryan R (2008) Recordings or summaries of consultations for people with cancer. Cochrane Database Syst Rev (3): CD001539.
Ptacek JT, McIntosh EG (2009) Physician challenges in communicating bad news. J Behav Med 32 (4): 380–387.
Rodin G, Mackay JA, Zimmermann C, Mayer C, Howell D, Katz M, Sussman J, Browers M (2009) Clinician-patient communication: a systematic review. Support Cancer Care 17: 627–644.
Schofield PE, Butow PN, Thompson JF, Tattersall MHN, Beeney LJ (2003) Psychological responses of patients receiving a diagnosis of cancer. Ann Oncol 14 (1): 48–56.
Short D, Frischer M, Bashford J . Barriers to the adoption of computerised decision support systems in general practice consultations: a qualitative study of GPs’ perspectives. Int J Med Inform (2004) ; 73 (4): 357–362.
Silvia KA, Ozanne EM, Sepucha KR (2008) Implementing breast cancer decision aids in community sites: barriers and resources. Health Expect 11 (1): 46–53.
Smith JA, Flowers P, Larkin M (2009) Interpretive phenomenological analysis. Theory, Method and Research. Sage Publications: London.
Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Légaré F, Thomson RO (2011) Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 10, CD001431.
Stacey D, Kryworuchko J, Bennett C, Murray MA, Mullan S, Légaré F (2012) Decision coaching to prepare patients for making health decisions: a systematic review of decision coaching in trials of patient decision aids. Med Decis Making 32: 22–33.
Takeuchi EE, Keding A, Awad N, Hofmann U, Campbell LJ, Selby PJ, Brown JM, Velikova G (2011) Impact of patient-reported outcomes in oncology: a longitudinal analysis of patient-physician communication. J Clin Oncol 29 (21): 2910–2917.
Towle A, Godolphin W, Manklow J, Wiesinger H (2003) Patient perceptions that limit a community-based intervention to promote participation. Patient Educ Counsel 50 (3): 231–233.
Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, Selby PJ (2004) Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 22 (4): 714–724.
Acknowledgements
We are indebted to the patients and doctors who took part in this study. We are very grateful to our Patient and Carer Study Group who have been a major force behind this project and have invested a large amount of time bringing this to fruition. Finally, we would like to acknowledge funding from the National Institute for Health Research (NIHR) under its Research for Patient Benefit Programme (Grant reference number PB-PG-0807-14122). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Furber, L., Murtagh, G., Bonas, S. et al. Improving consultations in oncology: the development of a novel consultation aid. Br J Cancer 110, 1101–1109 (2014). https://doi.org/10.1038/bjc.2013.749
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.749
Keywords
This article is cited by
-
Concerns raised by people treated for head and neck cancer: a secondary analysis of audiotaped consultations in a health services follow-up clinic
Supportive Care in Cancer (2023)
-
Potential curability and perception of received information in esophageal cancer patients
Supportive Care in Cancer (2018)