Abstract
Background:
This study is to investigate the effects of geserelin+tamoxifen (TAM) on estradiol level, breast density (BD), endometrial thickness (ET), and blood lipids in premenopausal and perimenopausal women with hormone receptor-positive early-stage breast cancer.
Methods:
This study recruited 110 premenopausal and perimenopausal patients with hormone receptor-positive early-stage breast cancer between 22 June 2008 and 31 December 2009 and randomly assigned them to receive either goserelin plus TAM or TAM alone for 1.5 years. Blood levels of sex hormones and lipids and ET were determined at 0, 3, 6, 12, and 18 months. Contralateral BD was also measured at 0, 12, and 18 months.
Results:
Five participants dropped out of the goserelin plus TAM group, and two participants dropped out of the TAM-alone group before initiation of endocrine therapy. The rest of patients received scheduled treatment and 3 years of median follow-up. No serious adverse effects were observed, and only two local recurrences have been observed in these patients. Estradiol level and BD were lower in the goserelin plus TAM group than in the TAM-alone group (P<0.05). The endometrium in the goserelin plus TAM group was significantly thinner than that in the TAM-alone group (P<0.05), and women in the TAM-alone group exhibited endometrial thickening over the course of the study. Furthermore, no significant differences in blood lipid levels were reported between the two groups.
Conclusion:
The data from the current study demonstrated that the addition of goserelin to TAM results in downregulation of estradiol level, followed by significant reduction in BD and ET in premenopausal and perimenopausal women with hormone receptor-positive breast cancer, which may eventually lead to better outcome in these patients.
Similar content being viewed by others
Main
Breast cancer is a kind of sex hormone-dependent disease. Factors that contribute to the international variation in incidence rates largely stem from the availability of early-detection services as well as differences in reproductive and hormonal factors, namely, risk factors associated with the level and duration of exposure to estrogen (Trialists’ Collaborative Group (EBCTCG), 2005). Based on several large clinical trials, estrogen blockade agents and several aromatase inhibitors (AIs) have become the standard adjuvant treatment for postmenopausal patients with endocrine-responsive early-breast cancer (Coombes et al, 2004; Breast International Group (BIG) 1-98 Collaborative Group, 2005; Howell et al, 2005; Coates et al, 2007). In the past two decades, medical castration with luteinising hormone-releasing hormone agonists (LHRHa’s) has been proposed in clinical practice. A frequently used LHRHa, goserelin, acts on the hypothalamic–pituitary axis and achieves reversible ovarian function suppression through receptor downregulation, and reduces luteinising hormone and estradiol to postmenopausal levels (Goel et al, 2009; Sverrisdottir et al, 2011). Results from randomised clinical trials illustrated that the use of LHRHa did improve the survival duration of patients with hormone receptor-positive (estrogen receptor-positive (ER+) and/or progesterone receptor-positive (PR+)) breast cancers (Soreide et al, 2002; Baum et al, 2006; Cuzick et al, 2007). To elaborate the role of suppression of ovarian function in premenopausal patients with endocrine-responsive breast cancer more clearly, three trials were sponsored by the International Breast Cancer Study Group and coordinators. The results of SOFT/TEXT/PERCHE trials are awaited to answer these questions: the role of OFS, the role of AI in combination with OFS, and the role of chemotherapy in addition to endocrine therapies (Regan et al, 2008).
As a kind of endocrine therapy, OFS can suppress breast cancer and improve the survival of premenopausal patients directly. Besides, OFS may also improve the survival of premenopausal patients with hormone receptor-positive breast cancer indirectly by decreasing the contralateral breast density (BD), endometrial thickness (ET), and so on. An initial BIG 1-98 study reported that postmenopausal breast cancer patients who received TAM had an increased incidence of thromboembolic events and endometrial cancer, although TAM had the predominant benefit on disease-free survival (DFS) and overall survival (OS), with a proportional reduction in contralateral breast cancer (BIG 1-98 Collaborative Group, 2009; Colleoni et al, 2011). The result of a subgroup analysis of the IBIS I Breast Cancer Prevention Trial showed that the discontinuation rate in the TAM group was high and occurred early because of gynaecological findings, most notable of which was the finding that in postmenopausal women, the median ET was significantly increased within 5 years in the TAM group (Palva et al, 2012). In contrast, goserelin was not associated with any significant thromboembolic events or with the occurrence of endometrial cancer (Cheer et al, 2005). Intriguingly, goserelin significantly decreased the mammographic BD in patients carrying a BRCA1 mutation, suggesting that goserelin may repress breast cancer recurrence by reducing mammary density. It was also confirmed that the reduction in BD apparently reduced the risk of contralateral breast cancer (Cuzick et al, 2011).
Therefore, in this study, we hypothesised that the combination of goserelin with TAM could lead to downregulation of the estradiol level, resulting in reduced BD and ET, and ultimately leading to better DFS and OS in premenopausal and perimenopausal patients with hormone receptor-positive breast cancer. Thus, we initiated a randomised controlled trial that was sponsored by the Zhejiang Cancer Hospital in 2008 to validate our hypothesis.
Patients and Methods
Participants and study design
In this study, we recruited 110 premenopausal or perimenopausal women with hormone receptor-positive early-stage breast cancers who had undergone radical mastectomy or breast-conserving surgery at the Zhejiang Cancer Hospital in Hangzhou, China, between 22 June 2008 and 31 December 2009. Hormone receptor-positive means ER+ and/or PR+. The patients were randomly assigned to one of the two groups: the goserelin plus TAM group (n=56) or the TAM-alone group (n=54). Before receiving any endocrine therapy, these patients underwent surgery, followed by various radiochemotherapy regimens (Table 1) after evaluation by a multidisciplinary team consisting of a medical oncologist, radiation oncologist, and oncological surgeon. The data on the patients’ hormone receptor status and pathological diagnoses were obtained from the Department of Pathology, and clinical data were obtained from medical records. The Ethics Committee and the Academic Committee of Zhejiang Cancer Hospital approved this clinical study. Written informed consent was obtained from all patients for our study before trial entry. Both TAM (Nolvadex) and goserelin (Zoladex) were supplied by Astra-Zeneca China Co, Ltd (Shanghai, China). This study is registered, number NCT00827307.
Inclusion and exclusion criteria
Patients with hormone receptor-positive early-stage breast cancers were included in the trial if there was no clinical evidence of metastatic or recurrent diseases, and if they were histopathologically confirmed as having stage-I/II ER+ and/or PR+-invasive breast cancer. The standard of hormone receptor-positive was ER ⩾10% and/or PR ⩾10% by immunohistochemistry stain. All patients were then characterised as premenopausal or perimenopausal (last menstruation <6 months before trial entry) according to the National Comprehensive Cancer Network guidelines. Temporary chemotherapy-induced amenorrhea was allowed, provided that the premenopausal status was confirmed by estradiol level within 8 months before the final dose of chemotherapy. These patients were also required to be tested to ensure that they had adequate haematological reserves, renal function, and liver function.
The exclusion criteria were as follows: women with a life-threatening concurrent disease were excluded from the study. Pregnant and lactating women with early-stage breast cancer and patients with distant metastasis were ineligible for this study. Patients who had had intermissions of 8 weeks or longer from the time of surgery to postoperative chemotherapy, or from chemotherapy completion to study recruitment, were rejected according to the clinical protocol. In addition, those who had a history of previous endocrine treatment, bilateral ovarian radiation or excision, a malignant tumour in the past 5 years, and/or an allergy to goserelin or TAM were excluded from our study.
Patients were randomised after enrolment into this study. Treatment allocation was based on the permuted block technique. The voluntary aspect of the study was stressed, and confidentiality was guaranteed. All parameters of the two groups were comparable.
Intervention
Patients were randomly assigned to receive either 20 mg of TAM alone (1 × 10-mg tablet orally twice daily) or 20 mg of TAM (1 × 10-mg tablet orally twice daily) plus 3.6 mg of goserelin (subcutaneous injection every 28 days). The treatment duration was 1.5 years, and treatment would be continued in the adjuvant setting for both treatment groups for at least 5 years. The adverse effects of the endocrine drugs were documented, and compliance was assessed with an interview at each follow-up visit. According to the adverse effect criteria of the Eastern Cooperative Oncology Group (ECOG), intermittent endocrine treatment was optional if a patient presented with life-threatening adverse effects.
Follow-up and outcome measures
All baseline data were collected before endocrine treatment. Blood levels of sex hormones (estradiol, progesterone, and human chorionic gonadotropin) and lipids (triglycerides, cholesterol, low-density lipoprotein, and high-density lipoprotein), and ultrasonic gynaecological examinations were performed at 3, 6, 12, and 18 months after initiating endocrine treatment. The minimum detectable value of estradiol by ELISA was 1.0 pg ml−1, with a sensitivity of 99%. Patients underwent contralateral mammography at 12 and 18 months after endocrine treatment initiation. Routine haematologic and clinical chemistry measurements were performed at every follow-up visit.
The primary end point was estradiol level, mammographic BD, ultrasonic ET, and blood lipid levels. Blood lipid and sex hormone levels were measured in the clinical laboratory, which is the central laboratory of Zhejiang province. Mammographic images performed in our hospital were all digitalised, and BD was quantitatively measured on craniocaudal images of the unaffected breast with the use of R2 quantra (Hologic Inc, Bedford, MA, USA) by a single radiologist. ET was measured by a specified sonologist.
Statistical analysis
The estimated sample size was 48 in each group. To allow for 10% patient ineligibility, we set the sample size of our study at 110. Data analysis was carried out by using the SPSS 17.0 software (SPSS, Chicago, IL, USA). The quantified baseline data recorded in our study were compared between the two groups by using the Student’s t-test, and qualified data were compared by the Fisher’s exact test. The means of repeated measures were compared using a general linear model. P<0.05 was considered statistically significant. All P-values were two-sided.
Results
Characteristics of patients
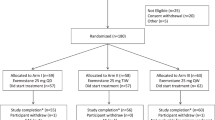
In this study, we recruited 110 patients with early-stage hormone receptor-positive breast cancer to receive goserelin and TAM or TAM-alone therapy. Of these patients, 7 (6.36%, 5 in goserelin plus TAM group and 2 in TAM-alone group) declined to participate after the randomised assignment, resulting in continued administration of goserelin plus TAM or TAM alone in 103 women (51 in goserelin plus TAM group and 52 in TAM-alone group) (Figure 1). Moreover, one in TAM-alone group did not completely finish the assigned intervention. The median duration of follow-up was 3 years. In both groups of patients, the median ages at the time of diagnosis and at the time of menarche were 42 and 15 years, respectively. The body mass index was similar between these two groups (P>0.05; Table 1). Baseline data of the end point events, including BD, ET, and blood lipid levels, were not significantly different (P>0.05; Table 1). Baseline data of blood sex hormone levels in premenopausal and perimenopausal women indicated that the ovarian function of women in group A was comparable to that in group B (P>0.05; Table 1).
Adverse effects and recurrent events
In this study, serious adverse effects were not observed during the period of intervention and follow-up. To date, all patients were alive and two had local recurrences.
Multivariance analysis of the end point events
A statistical Mauchly’s test was performed to validate the sphericity symmetry hypothesis for all measures, including estradiol, BD, ET, and blood lipid levels, but the results were negative (P<0.05). Thus, the Greenhouse–Geisser multivariance analysis was used to compare the repeated measures, and the results are shown in Table 2 and Figure 2. As expected, estradiol levels were remarkably repressed in goserelin plus TAM group relative to TAM-alone group (F=9.949; P=0.002), whereas blood lipid levels were not apparently altered after addition of goserelin (P>0.05). Compared with the use of TAM alone, the addition of goserelin to TAM significantly decreased BD (F=6.172; P=0.015). In parallel, the endometrium of patients assigned to receive goserelin plus TAM was clearly thinner compared with that of patients who received TAM-alone treatment (F=22.671; P<0.001).
Variation trends of breast density, endometrial thickness, estradiol (E2), and lipidemia. Note: Group (A), geserelin plus tamoxifen; group (B), tamoxifen alone. (A), E2; (B), breast density; (C), endometrial thickness; (D), triglycerides (TG); (E), Chol, cholesterol; (F), low-density lipoprotein (LDL); (G), high-density lipoprotein (HDL).
Moreover, a time-dependent trend was detected in the variation of BD according to a within-subject variance analysis (F=90.371; P<0.001), whereas interactive effects between timing and groups were not observed (F=0.633; P=0.483). The multivariate analysis showed that after the 12-month follow-up, BD was significantly attenuated in the goserelin plus TAM group compared with the TAM-alone group (P<0.05, Table 3). Bonferroni statistical data showed that after the 18-month follow-up, ET in the TAM-alone group was thicker than ET in the baseline data (mean difference =–0.751; P<0.001). There was no significant difference in ET in the goserelin plus TAM group among the various time points, although a downward trend was noted (mean difference =–0.301; P=0.057).
Discussion
Adjuvant endocrine therapy, which is an integral component in the treatment of patients with ER+ and/or PR+ breast cancer, exerts its effect by reducing the availability of estrogen to micrometastatic tumour cells (Goss et al, 2003; BIG 1-98 Collaborative Group, 2009; van de Velde et al, 2010). Thus TAM, the most commonly used hormonal treatment for breast cancer patients, was introduced into clinical practice in the 1970s and has substantially improved survival duration in patients with hormone receptor-positive breast cancers (Jaiyesimi et al, 1995; O’Regan and Jordan, 2002; Breast International Group (BIG) 1-98 Collaborative Group et al, 2005; Clarke, 2008; Rose, 2008; Masuda et al, 2012). However, TAM is also able to produce notable estrogenic effects on the skeletal system, lipid metabolism, and various gynaecological tissues, including mammary gland, vaginal mucosa, and uterus (Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group, 2006; Rose, 2008; Melnikow, 2010). Although TAM can improve survival duration in patients with estrogen-sensitive breast cancer, its long-term use inevitably increases the risk of adverse effects, such as endometrial cancer and disorders of lipid metabolism (Gardner et al, 2000; Nystedt et al, 2000; Weitzel et al, 2007). Routine screening of women without symptoms who are taking TAM has been suggested; however, this would not be cost-effective and would not likely lower mortality (Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group, 2006; Palva et al, 2012). Thus, an alternative strategy to address TAM-induced adverse effects and symptoms is to render the gynaecological tissues incapable of responding to estrogenic stimulation by using a LHRHa, such as goserelin (Sverrisdottir et al, 2011). Results from recent clinical trials support the clinical benefit of LHRHa’s in terms of DFS and/or OS (Jonat et al, 1995; Jonat, 1998; Nystedt et al, 2000; Kaufmann et al, 2003; Cheer et al, 2005; De Placido et al, 2005; Davidson et al, 2005), whereas Goel et al, 2009 pointed out in the Cochrane system review that there were insufficient data to compare the combinations of LHRHa’s plus TAM with TAM alone. Thus, in this study, we designed a randomised controlled trial to compare the effects of combined goserelin plus TAM with TAM alone on estradiol, BD, ET, and lipids in premenopausal and perimenopausal women with early-stage hormone receptor-positive breast cancer.
We found that there was a significant difference in trends of estradiol levels between these two groups of patients after endocrine therapy. The average estradiol level in the TAM-alone group reached a summit within the first 3 months, which may have been caused by the accumulation of estrogen related to TAM, and then decreased by degrees and ultimately tended to stabilise at a high level. In contrast, the average estradiol level in the goserelin plus TAM group remained low and stable. Obviously, goserelin reduced the estradiol level when added to the TAM treatment.
Moreover, variation tendencies of BD and ET were statistically different between the two groups of patients after endocrine therapy. In both groups, a trend in descending BD was noted, but the average BD value in the goserelin plus TAM group was lower than that in the TAM-alone group. Breast density in the goserelin plus TAM group decreased more than in the TAM-alone group, indicating that TAM-induced BD reduction was enhanced by goserelin. Moreover, TAM-induced endometrial thickening was efficiently blocked by the addition of goserelin into the endocrine regimen. The average amount of ET was lower and was more stable in the goserelin plus TAM group than in the TAM-only group, where the average ET tended to increase. These data indicate that the effects of goserelin on ET and BD were due to goserelin-induced estradiol changes, which in turn prevented hyperplasia of mammary ductal, glandular, and endometrial epithelia.
Furthermore, our current study showed that goserelin did not seem to affect lipid metabolism in the study patients, which was supported by similar blood lipid levels observed in patients from both the goserelin plus TAM group and the TAM-alone group. After a 3-year follow-up, all patients were alive and there were only two recurrent events. Although the effect of combined goserelin and TAM on DFS and OS in premenopausal patients with stages I and II breast cancer was significantly validated by the Austrian Breast and Colorectal Cancer Study Group Trial 5 (Jakesz et al, 2002), we were unable to determine conclusively whether better DFS and OS were reached because of the small sample size and short follow-up time of our study. However, our outcomes still partially reflected the great effects of goserelin on estradiol level, mammography BD, and ET against TAM in premenopausal and perimenopausal women with hormone receptor-positive breast cancer. A Korean study showed that mammography BD change during short-term use of adjuvant endocrine therapy was a significant predictor of long-term recurrence in women with ER+ breast cancer (Kim et al, 2012). We speculate that goserelin combined with TAM may lead to better DFS and OS in premenopausal and perimenopausal women with hormone receptor-positive early-stage breast cancer than would be seen with the use of TAM alone through downregulation of estrogen and reduction of BD and ET. Further follow-up for this study is still continuing. However, a randomised trial with a larger sample size and longer duration of follow-up is subsequently needed to confirm these results.
Change history
06 August 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group,, Buzdar A, Howell A, Cuzick J, Wale C, Distler W, Hoctin-Boes G, Houghton J, Locker GY, Nabholtz JM (2006) Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 7: 633–643.
Baum M, Hackshaw A, Houghton J, Rutqvist Fornander T, Nordenskjold B, Nicolucci A, Sainsbury R ZIPP International Collaborators Group (2006) Adjuvant goserelin in pre-menopausal patients with early breast cancer: results from the ZIPP study. Eur J Cancer 42: 895–904.
BIG 1-98 Collaborative Group,, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Goldhirsch A, Thürlimann B, Paridaens R, Smith I, Mauriac L, Forbes JF, Price KN, Regan MM, Gelber RD, Coates AS (2009) Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 361: 766–776.
Breast International Group (BIG) 1-98 Collaborative Group, Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353: 2747–2757.
Cheer SM, Plosker GL, Simpson D, Wagstaff AJ (2005) Goserelin: a review of its use in the treatment of early breast cancer in premenopausal and perimenopausal women. Drugs 65: 2639–2655.
Clarke MJ (2008) WITHDRAWN: tamoxifen for early breast cancer. Cochrane Database Syst Rev 8: CD000486.
Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Láng I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 25: 486–492.
Colleoni M, Giobbie-Hurder A, Regan MM, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Láng I, Smith I, Chirgwin J, Pienkowski T, Wardley A, Price KN, Gelber RD, Coates AS, Goldhirsch A (2011) Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 Study. J Clin Oncol 29: 1117–1124.
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C Intergroup Exemestane Study (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350: 1081–1092.
Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M, Sainsbury R (2007) Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 369: 1711–1723.
Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM (2011) Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case–control study. J Natl Cancer Inst 103: 744–752.
Davidson NE, O'Neill AM, Vukov AM, Osborne CK, Martino S, White DR, Abeloff MD (2005) Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188). J Clin Oncol 23: 5973–5982.
De Placido S, De Laurentiis M, De Lena M, Lorusso V, Paradiso A, D'Aprile M, Pistillucci G, Farris A, Sarobba MG, Palazzo S, Manzione L, Adamo V, Palmeri S, Ferraù F, Lauria R, Pagliarulo C, Petrella G, Limite G, Costanzo R, Bianco AR GOCSI Cooperative Group (2005) A randomised factorial trial of sequential doxorubicin and CMF vs CMF and chemotherapy alone vs chemotherapy followed by goserelin plus tamoxifen as adjuvant treatment of node-positive breast cancer. Br J Cancer 92: 467–474.
Gardner FJ, Konje JC, Abrams KR, Brown LJ, Khanna S, Al-Azzawi F, Bell SC, Taylor DJ (2000) Endometrial protection from tamoxifen-stimulated changes by a levonorgestrel-releasing intrauterine system: a randomised controlled trial. Lancet 356: 1711–1717.
Goel S, Sharma R, Hamilton A, Beith J (2009) LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database Syst Rev 7: CD004562.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349: 1793–1802.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS ATAC Trialists’ Group (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365: 60–62.
Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN (1995) Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol 13: 513–529.
Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, Seifert M, Haider K, Mlineritsch B, Steindorfer P, Kwasny W, Fridrik M Austrian Breast and Colorectal Cancer Study Group Trial 5 (2002) Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer--Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol 20: 4621–4627.
Jonat W (1998) Luteinizing hormone-releasing hormone analogues--the rationale for adjuvant use in premenopausal women with early breast cancer. Br J Cancer 78 (Suppl 4): 5–8.
Jonat W, Kaufmann M, Blamey RW, Howell A, Collins JP, Coates A, Eiermann W, Jänicke F, Njordenskold B, Forbes JF, Kolvenbag G.J.C.M. (1995) A randomised study to compare the effect of the luteinising hormone releasing hormone (LHRH) analogue goserelin with or without tamoxifen in pre- and perimenopausal patients with advanced breast cancer. Eur J Cancer 31A: 137–142.
Kaufmann M, Jonat W, Blamey R, Cuzick J, Namer M, Fogelman I, de Haes JC, Schumacher M, Sauerbrei W Zoladex Early Breast Cancer Research Association(ZEBRA) Trialists’ Group (2003) Survival analyses from the ZEBRA study. goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer. Eur J Cancer 39: 1711–1717.
Kim J, Han W, Moon HG, Ahn SK, Shin HC, You JM, Han SW, Im SA, Kim TY, Koo HR, Chang JM, Cho N, Moon WK, Noh DY (2012) Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res 14: R102.
Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, Nishimura R, Iwase H, Kamigaki S, Takei H, Noguchi S (2012) Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol 13: 345–352.
Melnikow J (2010) ACP Journal Club Review: Tamoxifen, raloxifene, and tibolone prevent primary invasive breast cancer but increase risk for adverse outcomes. Ann Intern Med 152: JC3–JC4.
Nystedt M, Berglund G, Bolund C, Brandberg Y, Fornander T, Rutqvist LE (2000) Randomized trial of adjuvant tamoxifen and/or goserelin in premenopausal breast cancer--self-rated physiological effects and symptoms. Acta Oncol 39: 959–968.
O’Regan RM, Jordan VC (2002) The evolution of tamoxifen therapy in breast cancer: selective oestrogen-receptor modulators and downregulators. Lancet Oncol 3: 207–214.
Palva T, Ranta H, Koivisto AM, Pylkkänen L, Cuzick J, Holli K (2012) A double-blind placebo-controlled study to evaluate endometrial safety and gynaecological symptoms in women treated for up to 5 years with tamoxifen or placebo—A substudy for IBIS I Breast Cancer Prevention Trial. Eur J Cancer 49: 45–51.
Regan MM, Pagani O, Walley B, Torrisi R, Perez EA, Francis P, Fleming GF, Price KN, Thürlimann B, Maibach R, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Gelber RD SOFT/TEXT/PERCHE Steering Committee; International Breast Cancer Study Group (2008) Premenopausal endocrine-responsive early breast cancer: who receives chemotherapy? Ann Oncol 19: 1231–1241.
Rose C (2008) Increasing protection after tamoxifen: insights from the extended adjuvant aromatase inhibitor trials. J Cancer Res Clin Oncol 134: 7–17.
Soreide JA, Varhaug JE, Fjosne HE, Erikstein B, Jacobsen AB, Skovlund E, Kvinnsland S (2002) Adjuvant endocrine treatment (goserelin vs tamoxifen) in pre-menopausal patients with operable node positive stage II breast cancer. A prospective randomized national multicenter study. Eur J Surg Oncol 28: 505–510.
Sverrisdottir A, Johansson H, Johansson U, Bergh J, Rotstein S, Rutqvist L, Fornander T (2011) Interaction between goserelin and tamoxifen in a prospective randomised clinical trial of adjuvant endocrine therapy in premenopausal breast cancer. Breast Cancer Res Treat 128: 755–763.
Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717.
van de Velde CJ, Verma S, van Nes JG, Masterman C, Pritchard KI (2010) Switching from tamoxifen to aromatase inhibitors for adjuvant endocrine therapy in postmenopausal patients with early breast cancer. Cancer Treat Rev 36: 54–62.
Weitzel JN, Buys SS, Sherman WH, Daniels A, Ursin G, Daniels JR, MacDonald DJ, Blazer KR, Pike MC, Spicer DV (2007) Reduced mammographic density with use of a gonadotropin-releasing hormone agonist-based chemoprevention regimen in BRCA1 carriers. Clin Cancer Res 13: 654–658.
Acknowledgements
We thank Astra-Zeneca Co, Ltd and The Chinese Anti-Cancer Association for funding this study (NCT00827307). We thank the patients and families who generously provided follow-up information beyond that available in our records. We also thank our clinical colleagues and support staff for helping us to acquire clinical data. We thank Dr Xiaochun Xu for editing this paper. This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yang, H., Zong, X., Yu, Y. et al. Combined effects of goserelin and tamoxifen on estradiol level, breast density, and endometrial thickness in premenopausal and perimenopausal women with early-stage hormone receptor-positive breast cancer: a randomised controlled clinical trial. Br J Cancer 109, 582–588 (2013). https://doi.org/10.1038/bjc.2013.324
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.324
Keywords
This article is cited by
-
Comparison of changes in lipid profiles of premenopausal women with early-stage breast cancer treated with different endocrine therapies
Scientific Reports (2022)
-
Impact of systemic adjuvant therapy and CYP2D6 activity on mammographic density in a cohort of tamoxifen-treated breast cancer patients
Breast Cancer Research and Treatment (2021)
-
Evaluating the Survival Benefit Following Ovarian Function Suppression in Premenopausal Patients with Hormone Receptor Positive Early Breast Cancer
Scientific Reports (2016)