Abstract
Background:
Body mass index (BMI) has been associated with the risk of oesophageal cancer. But the influence of BMI on postoperative complication and prognosis has always been controversial.
Methods:
In total, 2031 consecutive patients who underwent oesophagectomy between 1998 and 2008 were classified according to Asian-specific BMI (kg m−2) cutoff values. The impact of BMI on overall survival (OS) was estimated using the Kaplan–Meier method and Cox proportional hazard models. We performed a meta-analysis to examine the association of BMI with OS and postoperative complication.
Results:
Patients with higher BMI had more postoperative complication (P=0.002), such as anastomotic leakage (P=0.016) and cardiovascular diseases (P<0.001), but less incidence of chylous leakage (P=0.010). Logistic regression analysis showed that BMI (P=0.005) was a confounding factor associated with postoperative complication. Multivariate analysis showed that overweight and obese patients had a more favourable survival than normal weight patients (HR (hazard ratio) = 0.80, 95% CI (confidence interval): 0.70–0.92, P=0.001). Subgroup analysis showed that the association with higher BMI and increased OS was observed in patients with oesophageal squamous cell carcinoma (ESCC) (P<0.001), oesophageal adenocarcinoma (EA) (P=0.034), never-smoking (P=0.035), ever-smoking (P=0.035), never alcohol consumption (P=0.005), weight loss (P=0.003) and advanced pathological stage (P<0.001). The meta-analysis further corroborated that higher BMI was associated with increased complication of anastomotic leakage (RR (risk ratio)=1.04, 95% CI: 1.02–1.06, P=0.001), wound infection (RR=1.03, 95% CI: 1.00–1.05, P=0.031) and cardiovascular diseases (RR=1.02, 95% CI: 1.00–1.05, P=0.039), but decreased incidence of chylous leakage (RR=0.98, 95% CI: 0.96–0.99, P<0.001). In addition, high BMI could significantly improved OS (HR=0.78, 95% CI: 0.71–0.85, P<0.001).
Conclusion:
Preoperative BMI was an independent prognostic factor for survival, and strongly associated with postoperative complications in oesophageal cancer.
Similar content being viewed by others
Main
Oesophageal cancer is one of the most common cancers in the world, with >480 000 new cases and 400 000 deaths annually, of which about half occurred in China (Jemal et al, 2011). Despite advances of surgical techniques and incorporation of new therapeutic approaches, oesophageal cancer is still a highly devastating disease with poor prognosis (van Hagen et al, 2012). There is a strong evidence that lifestyle factors such as physical activity, diet and obesity may have an effect on survival for some cancers (Davies et al, 2011).
The association between body mass index (BMI) and the risk of oesophageal cancer has been established (Oh et al, 2005; Tran et al, 2005; Kubo and Corley, 2006; Smith et al, 2008; Turati et al, 2012). However, there is no general consensus on the influence of BMI on survival in oesophageal cancer. Some studies suggested that patients with higher BMI had a significantly better prognosis than those with lower BMI (Smith et al, 2008; Hayashi et al, 2010; Melis et al, 2011; Kayani et al, 2012; Scarpa et al, 2012), whereas others yielded conflicting results (Healy et al, 2007; Morgan et al, 2007; Schumacher et al, 2009; Skipworth et al, 2009; Grotenhuis et al, 2010; Madani et al, 2010; Yoon et al, 2011; Blom et al, 2012). In addition, whether patients with higher BMI would have an increased incidence of postoperative complication is still debated. Several studies described no differences in postoperative complications after oesophagectomy (Morgan et al, 2007; Scipione et al, 2007; Melis et al, 2011; Blom et al, 2012), whereas some reported that a higher incidence of severe complications were noted in patients with higher BMI (Healy et al, 2007; Grotenhuis et al, 2010; Hayashi et al, 2010).
Therefore, we analysed a large cohort of Chinese patients with oesophageal cancer and carried out a comprehensive meta-analysis to elucidate these two controversial issues.
Materials and Methods
Patients
We identified consecutive patients with oesophageal cancer who underwent surgical resection at Sun Yat-sen University Cancer Center between December 1998 and December 2008. Patients were excluded if they received neoadjuvant or adjuvant therapy, had an unknown BMI or history of other cancer. Patient characteristics and postoperative complications were collected from retrospective medical record review using a standardized data collection form. Surgical procedure was performed as previously described in our studies (Liu et al, 2012). The most common surgical approaches included the left transthoracic procedure, the Ivor-Lewis approach and the cervicothoracoabdominal procedures. Lymph node dissection including standard or extended dissection of thoracic and abdominal lymph nodes was performed in patients with no evidence of metastatic disease that included cervical or coeliac lymph node metastases. Pathologic stage was determined according to the 7th edition AJCC staging system (Rice et al, 2010). The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center. All patients provided a written informed consent according to the ethical approval.
BMI value
Body mass index (kg m−2) was calculated based on a direct measurement of height and weight at diagnosis. Patients were asked whether they had weight loss when compared with their usual weight when their weight was measured at diagnosis. Patients were classified according to Asian-specific BMI cutoff value (Choi et al, 2013) as follows: underweight (<18.5 kg m−2); normal weight (18.5–22.9 kg m−2) (reference group); overweight and obese (⩾23.0 kg m−2). These values were chosen because there is evidence that excess risks of mortality from all-cause occur at lower BMI levels in Asians than in Caucasian (Wen et al, 2009). Besides, the mean BMI of Chinese population was relatively low (Smith et al, 2008).
Definition of postoperative complications
All complications from surgery to discharge from hospital were prospectively documented. Respiratory diseases complications consisted of pneumonia, respiratory failure. Pneumonia required positive sputum cultures or clear clinical and radiographic evidence of consolidation. Respiratory failure was defined as the requirement for mechanical ventilation for >24 h after surgery. Anastomotic leakage was defined as extravasation of water-soluble contrast medium documented by radiography. Chylous leakage was defined as the milky and elevated triglyceride level drained fluid. Wound infection was defined as purulent discharge from a closed surgical wound, with signs and symptoms of inflammation of the surrounding tissue together with abnormal smell. Vocal cord paresis was defined as hoarseness, pain in the throat when speaking and aspiration (due to poor swallowing reflex) with frequent resultant coughing. Cardiovascular diseases were defined as the myocardial infarction and arrhythmia detected by electrocardiogram.
The standardized manner of postoperative complications was reported according to the classification system composed by Dindo et al (2003). This system was based on the therapeutic consequences of complications and consists of five grades. Grading of complications was performed according to the most severe complication in each patient.
Statistical analysis
Statistical analysis was performed using the SPSS 16.0 for windows software system (SPSS Inc., Chicago, IL, USA). Differences between three groups were tested by the Kruskal–Wallis test. The association between BMI categories and clinicopathologic parameters or postoperative complication was analysed by χ2-square test or Fisher’s exact test. Follow-up time was calculated from the date of surgery to the event or date of the last contact. Follow-up continued until June 2012.The primary endpoint was overall survival (OS), which was calculated from the time of surgery to the time of death from any causes. The second endpoints were postoperative complication and disease-free survival (DFS). Disease-free survival was calculated from the time from surgery to the first recurrence of index cancer or to all-cause death. Multivariate logistic regression models were used to account for potential confounding factors associated with postoperative complication. Survival curves were calculated by the Kaplan–Meier method and analysed by log-rank test. Multivariate analysis was performed using Cox’s proportional hazards regression model with a forward stepwise procedure (the entry and removal probabilities were 0.05 and 0.10, respectively). A significant difference was declared if the P-value from a two-tailed test was <0.05.
Meta-analysis
Two reviewers independently performed systematic literature search of the following databases: PubMed, Embase, Web of Science and CNKI database (last search up to December 2012). The following search terms were used: ‘oesophageal cancer or oesophageal neoplasms’, ‘body mass index or overweight or obesity’ and ‘survival or prognosis’. All potentially eligible studies were retrieved. Studies were included if they met all of the following criteria: (1) patients with oesophageal cancer who underwent surgery, (2) BMI as an exposure interest, (3) information provided for estimating parameters and (4) published in English, German and Chinese with English abstract. Disagreements between reviewers were resolved by a third reviewer or by discussion and consensus. We assessed and quantified statistical heterogeneity for each pooled estimate using the I2 statistic. If heterogeneity existed, a random effects model was adopted; otherwise, a fixed effects model was used. Pooled analysis was performed using the Mantel–Haenszel model and reported as hazard ratio (HR) with 95% confidence intervals (CIs) for the assessment of the influence of BMI on OS and risk ratio (RR) with 95% CIs for the association between BMI and postoperative complication. Where possible, the HR and associated variance were obtained directly from each study. When the association between BMI and HRs of survival was not reported, HRs were calculated by the methods of Parmer et al (1998) and Tierney et al (2007). The Begg’s funnel plot and Egger’s test were employed to estimate the potential publication bias. Sensitivity analysis was conducted to re-evaluate the overall results by omitting specific studies. The significance of the pooled HR or RR was determined by the Z-test and P<0.05 was considered as statistical significance. All analyses were performed with the software STATA version 12 (StataCorp, College Station, TX, USA).
Results
Patient characteristics by BMI
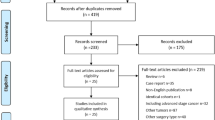
After excluding patients who receiving neoadjuvant or adjuvant therapy or with unknown BMI, 2031 consecutive patients with oesophageal cancer were included in the study and were divided into three groups according to BMI (Figure 1). Patient characteristics were shown in Table 1. Patients with higher BMI were more likely to be diagnosed with oesophageal adenocarcinoma (EA) and less likely to be oesophageal squamous cell carcinoma (ESCC) (P=0.003). Besides, overweight and obese patients were less likely to be smoker (P<0.001) and alcohol consumers (P=0.044), had lower likelihood of weight loss (P<0.001) when compared with normal weight.
Postoperative complication
With respect to perioperative complication, overweight and obese patients had more postoperative complication (P=0.002), such as anastomotic leakage (P=0.016) and cardiovascular disease (P<0.001). In addition, they had a longer operative time (P<0.001) than those with normal weight. Interestingly, overweight and obesity was associated with less incidence of chylous leakage in comparison to normal weight (P=0.010). There was significant difference in the rate of postoperative complication for different surgical procedures, 29.2% for cervicothoracoabdominal procedure, 12% for the Ivor-Lewis and 8% for the left transthoracic procedure (P<0.001) (data not shown). Logistic regression analysis showed that BMI (P=0.005), surgical procedures (P<0.001) and age (P=0.046) were confounding factors associated with postoperative complication (Supplementary Table 1). When all postoperative complications were categorised according to the Dindo classification, there were also significant differences between patients with underweight, normal weight, overweight and obesity (P=0.012, Table 1).
Univariate and multivariate analysis
The median of follow-up was 64 months. Univariate survival analysis showed a strongly significant difference in OS among three groups of patients. The 5-year OS and 10-year OS of patients with higher BMI were significantly longer than those of patients with lower BMI (P<0.001, Table 2, Figure 2). In addition, there was also significant difference in 5-year DFS among three groups of patients, 34.7% for underweight group, 37.3% for normal weight and 40.7% for overweight and obese (P=0.009, Supplementary Table 2). As given in Table 2, patients with old age, male, advanced pT category, lymph node metastasis, oesophagogastric junction tumour location, weight loss, a history of smoking and alcohol consumption and poor histologic differentiation were found to have a significantly shorter OS. In the final multivariate survival analysis with adjustment for covariates, we found that overweight and obese patients had a 20% lower risk of dying from any cause including oesophageal cancer when compared with normal weight patients (HR=0.80, 95% CI: 0.70–0.92, P=0.001).
In order to reduce possible effects of reverse causality due to prior diagnosed diseases or undiagnosed diseases, the sensitivity analyses were performed by excluding patients with prior diagnosed diseases (HR=0.86, 95% CI: 0.78–0.95, P=0.003) or died during the first 1 year of follow-up (HR=0.85, 95% CI: 0.78–0.92, P<0.001). The results did not substantially differ from the main results. We re-run the univariate survival analysis using a traditional BMI value 25 for overweight and obese instead of 23, and the increased OS for overweight and obese patients was also found (HR=0.83, 95% CI: 0.71–0.98, P=0.031) when compared with normal weight (BMI<25 kg m−2).
Subgroup analysis
Univariate survival analyses stratified by histology, smoking status, alcohol consumption, weight loss and pathological stage were performed. We found that the association with higher BMI and increased OS were observed in patients with ESCC (P<0.001), EA (P=0.034), never-smoking (P=0.035), ever-smoking (P=0.035), never alcohol consumption (P=0.005), weight loss (P=0.003) and advanced pathological stage (P<0.001) (Supplementary Table 3).
Meta-analysis of BMI and postoperative complication
As the search flow diagram showed (Figure 3), 14 studies including the current study, were included in our meta-analysis (Table 3) (Trivers et al, 2005; Healy et al, 2007; Morgan et al, 2007; Schumacher et al, 2009; Skipworth et al, 2009; Grotenhuis et al, 2010; Hayashi et al, 2010; Madani et al, 2010; Melis et al, 2011; Yoon et al, 2011; Zhu et al, 2011; Blom et al, 2012; Scarpa et al, 2012). Of the 14 studies, 7 studies were conducted in Europe, 4 in the United States, 2 in China and 1 in Canada, 1 study published in German, 1 in Chinese with English abstract and others were all in English. Only patients with oesophageal cancer in one study which enrolled both of oesophageal and gastric cancer were included in our meta-analysis (Trivers et al, 2005). Owing to the varied cutoff of BMI in each study, we pooled estimate of comparison of the highest BMI group with the lowest group for consistency (Table 3).
To evaluate the association of higher BMI with increased incidence of postoperative complication, several studies reporting postoperative complication were included. As shown in Supplementary Table 4, higher BMI was significantly associated with increased complication of anastomotic leakage (RR=1.04, 95% CI: 1.02–1.06, P=0.001, Figure 4a), wound infection (RR=1.03, 95% CI: 1.00–1.05, P=0.031, Figure 4b) and cardiovascular diseases (RR=1.02, 95% CI: 1.00–1.05, P=0.039, Figure 4c). More interestingly, patients with higher BMI inversely had a significantly decreased incidence of chylous leakage (RR=0.98, 95% CI: 0.96–0.99, P<0.001, Figure 4d). But with respect to the incidence of respiratory diseases and in-hospital mortality, there was no significant difference between the highest BMI group and lowest group. In all above pooled estimates, no significance of heterogeneity and publication bias was detected (Supplementary Table 4).
Meta-analysis of BMI and survival
All 14 studies were included to estimate the association of BMI and survival in oesophageal cancer. We found that patients with higher BMI had a significantly favourable OS (HR=0.78, 95% CI: 0.71–0.85, P<0.001, Figure 5a), there was no evidence of heterogeneity between the studies (P=0.188, I2=24.7%). The Begg’s funnel plots showed no evidence of obvious asymmetry (Supplementary Figure 1), and Egger’s test indicated no significance of publication bias (P>0.05). Sensitivity analysis was carried out to assess the influence of individual studies on the summary effect. Removal of one study published in German, one in Chinese with English and our current study, did not alter the overall result (HR=0.80, 95% CI: 0.72–0.89, P<0.001). To examine whether the association of higher BMI and increased OS was observed when using a traditional BMI cutoff value 25, five studies with the same BMI cutoff value 25 were included. We also found the similar result (HR=0.82, 95% CI: 0.72–0.94, P=0.004, Figure 5b).
Discussion
Body mass index has been associated with the risk of oesophageal cancer. Nevertheless, the effect of BMI on postoperative complication and prognosis of oesophageal cancer remains controversial. The main reasons can be summarised as follows: lack of large-scale clinical studies; different BMI cutoff values in different studies; some patients receiving neoadjuvant or adjuvant therapy were enrolled in some studies. Therefore, in our large-scale cohort study, patients were classified according to Asian-specific BMI cutoff values. In addition, patients who received neoadjuvant or adjuvant therapy were excluded. To our knowledge, our study consisting of 2031 Chinese patients cohort and meta-analysis was the first time to systematically elucidate the association of BMI with postoperative complication and prognosis in oesophageal cancer.
In our study, higher BMI was proved to be a risk factor for postoperative complication, such as anastomotic leakage and cardiovascular diseases. Previous studies reported that patients with higher BMI had a higher incidence of anastomotic leakage (Healy et al, 2007; Grotenhuis et al, 2010; Blom et al, 2012). The potential mechanisms might be summarised as follows: overweight and obese patients were performed a more challenge of a cervical anastomosis procedure and had higher rate of diabetes mellitus which could adversely affect the growth of anastomosis (Blom et al, 2012). Interestingly, we found that patients with higher BMI had less incidence of chylous leakage when compared with lower BMI. This result was in accordance with some previous studies (Morgan et al, 2007; Blom et al, 2012). However, the underlying mechanisms were rarely elucidated and in need to be further studied. The number of event for postoperative complication in each study was small and our results still needed further confirmation.
The clinical cohort study and meta-analysis both suggested that patients with higher BMI had a favourable survival when compared with lower BMI. In our cohort study, overweight and obese patients had an apparently longer 5-year OS than normal weight. Multivariate survival analysis showed that BMI was an independent prognostic factor in oesophageal cancer. Sensitivity analysis by excluding patients with prior diagnosed diseases or who died within the first 1 year of follow-up showed the similar result. Given most studies used a traditional BMI value 25 for overweight and obese, we re-run the univariate survival analysis using 25 as BMI cutoff. And we found that higher OS for overweight and obese patients was still noted. What is more, meta-analysis by pooling five studies with the same BMI cutoff value 25 confirmed this result. Our finding was similar to some previous studies (Smith et al, 2008; Hayashi et al, 2010; Melis et al, 2011; Scarpa et al, 2012), including one meta-analysis based on small sample size (Kayani et al, 2012). In addition, a survival advantage in patients with higher BMI has been repeatedly described for renal cancer and sporadically reported for gastric cancer (Dindo et al, 2003; Mullen et al, 2008).
The mechanism by which overweight and obese patients might improve survival is not well understood. The prognostic advantage for overweight and obese patients might be attributed to the fact that overweight and obese was associated with never-smoking, never alcohol consumption and no weight loss in our present study. All of these factors were proved to affect survival not only in our study but also in other studies (Thrift et al, 2012). Besides, patients with overweight and obese were more likely to be diagnosed with EA and less likely to be ESCC when compared with normal weight. Patients with EA were reported to have a better prognosis than those with ESCC (Holscher et al, 1995). However, when we performed univariate survival analyses stratified by smoking status, alcohol consumption, weight loss and histology, the association with higher BMI and increased OS were observed in patients with never-smoking, ever-smoking, never alcohol consumption, weight loss, ESCC and EA subgroup. The findings indicated that weight loss rather than smoking or alcohol consumption status or histology might be responsible for the survival difference. In essence, the decreased oesophageal cancer death leaded to the better prognosis for higher BMI patients because higher DFS for them was noted in our study.
In addition, a recent study indicated that preoperative nutritional deficiency was associated with poor survival in cancer patients (Morgan et al, 2011). Overweight and obese patients might have a better nutritional status and potential survival advantage because they had large appetites and high lipid concentration, and could adequately preserve their fat and muscle mass (Davos et al, 2003). We should acknowledge that the association between BMI and survival might be influenced by unmeasured confounding factors such as selection criteria and specially the socioeconomic status. Patients with overweight and obese were thought to be associated with higher income and higher education condition in China. They were more likely to receive chemotherapy and/or radiotherapy after recurrence than patients with lower BMI because of the financial support.
In conclusion, our larger scale Chinese cohort study and meta-analysis provided more definite and quantitative evidence that higher BMI was associated with favourable survival and some postoperative complications including anastomotic leakage, wound infection and cardiovascular diseases in oesophageal cancer.
Change history
26 November 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Blom RL, Lagarde SM, Klinkenbijl JH, Busch OR, van Berge Henegouwen MI (2012) A high body mass index in esophageal cancer patients does not influence postoperative outcome or long-term survival. Ann Surg Oncol 19: 766–771.
Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Adami HO, Lee JE, Lee HM (2013) Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer 132: 625–634.
Davies NJ, Batehup L, Thomas R (2011) The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer 105 (Suppl 1): S52–S73.
Davos CHDW, Rauchhaus M, Cicoira M, Francis DP, Coats AJ, Clark AL, Anker SD (2003) Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail 9: 29–35.
Dindo D, Muller MK, Weber M, Clavien PA (2003) Obesity in general elective surgery. Lancet 361: 2032–2035.
Grotenhuis BA, Wijnhoven BP, Hotte GJ, van der Stok EP, Tilanus HW, van Lanschot JJ (2010) Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg 34: 2621–2627.
Hayashi Y, Correa AM, Hofstetter WL, Vaporciyan AA, Rice DC, Walsh GL, Mehran RJ, Lee JH, Bhutani MS, Dekovich A, Swisher SG, Ajani JA (2010) The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer 116: 5619–5627.
Healy LA, Ryan AM, Gopinath B, Rowley S, Byrne PJ, Reynolds JV (2007) Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg 134: 1284–1291.
Holscher AH, Bollschweiler E, Schneider PM, Siewert JR (1995) Prognosis of early esophageal cancer. Comparison between adeno- and squamous cell carcinoma. Cancer 76: 178–186.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90.
Kayani B, Okabayashi K, Ashrafian H, Harling L, Rao C, Darzi A, Kitagawa Y, Athanasiou T, Zacharakis E (2012) Does obesity affect outcomes in patients undergoing esophagectomy for cancer? A meta-analysis. World J Surg 36: 1785–1795.
Kubo A, Corley DA (2006) Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 15: 872–878.
Liu J, Hu Y, Xie X, Fu J (2012) Subcarinal node metastasis in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 93: 423–427.
Madani K, Zhao R, Lim HJ, Casson SM, Casson AG (2010) Obesity is not associated with adverse outcome following surgical resection of oesophageal adenocarcinoma. Eur J Cardiothorac Surg 38: 604–608.
Melis M, Weber JM, McLoughlin JM, Siegel EM, Hoffe S, Shridhar R, Turaga KK, Dittrick G, Dean EM, Karl RC, Meredith KL (2011) An elevated body mass index does not reduce survival after esophagectomy for cancer. Ann Surg Oncol 18: 824–831.
Morgan MA, Lewis WG, Hopper AN, Escofet X, Harvard TJ, Brewster AE, Crosby TD, Roberts SA, Clark GW (2007) Prognostic significance of body mass indices for patients undergoing esophagectomy for cancer. Dis Esophagus 20: 29–35.
Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, Chang SS, Cookson MS, Herrell SD, Smith JA Jr, Clark PE (2011) Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol 59: 923–928.
Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, Moorman DW (2008) Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol 15: 2164–2172.
Oh SW, Yoon YS, Shin SA (2005) Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol 23: 4742–4754.
Parmer M, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834.
Rice TW, Blackstone EH, Rusch VW (2010) 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 17 (7): 1721–1724.
Scarpa M, Cagol M, Bettini S, Alfieri R, Carraro A, Cavallin F, Trevellin E, Saadeh LM, Ruol A, Vettor R, Ancona E, Castoro C (2012) Overweight patients operated on for cancer of the esophagus survive longer than normal-weight patients. J Gastrointest Surg 17: 218–227.
Schumacher G, Schlechtweg N, Chopra SS, Rosch T, Veltzke-Schlieker W, Thuss-Patience P, Schmidt SC, Neuhaus P (2009) Impact of the body mass index on the prognosis and complication rate after surgical resection of cancers at the oesophagogastric junction. Zentralbl Chir 134: 66–70.
Scipione CN, Chang AC, Pickens A, Lau CL, Orringer MB (2007) Transhiatal esophagectomy in the profoundly obese: implications and experience. Ann Thorac Surg 84: 376–382, discussion 383.
Skipworth J, Foster J, Raptis D, Hughes F (2009) The effect of preoperative weight loss and body mass index on postoperative outcome in patients with esophagogastric carcinoma. Dis Esophagus 22: 559–563.
Smith M, Zhou M, Whitlock G, Yang G, Offer A, Hui G, Peto R, Huang Z, Chen Z (2008) Esophageal cancer and body mass index: results from a prospective study of 220 000 men in China and a meta-analysis of published studies. Int J Cancer 122: 1604–1610.
Thrift AP, Nagle CM, Fahey PP, Russell A, Smithers BM, Watson DI, Whiteman DC (2012) The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer 131: E759–E768.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 1–16.
Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, Mark SD, Qiao YL, Taylor PR (2005) Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer 113 (3): 456–463.
Trivers KF, De Roos AJ, Gammon MD, Vaughan TL, Risch HA, Olshan AF, Schoenberg JB, Mayne ST, Dubrow R, Stanford JL, Abrahamson P, Rotterdam H, West AB, Fraumeni JF, Chow WH (2005) Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol 3: 225–230.
Turati F, Tramacere I, La Vecchia C, Negri E (2012) A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 24: 609–917.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A CROSS Group (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366: 2074–2084.
Wen CP, David Cheng TY, Tsai SP, Chan HT, Hsu HL, Hsu CC, Eriksen MP (2009) Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 12: 497–506.
Yoon HH, Lewis MA, Shi Q, Khan M, Cassivi SD, Diasio RB, Sinicrope FA (2011) Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol 29: 4561–4567.
Zhu X, Chen H, Gao L, Xu X, Zhang S (2011) Prognostic value of body mass index on the outcome after resection of esophageal cancer. Anhui Med Pharm J 15: 347–349.
Acknowledgements
We would like to thank the authors of the studies included in our manuscript. This work was supported by of Chinese Ministry of Health Key Program grant (No. 179).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Zhang, S., Yang, H., Luo, K. et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer 109, 2894–2903 (2013). https://doi.org/10.1038/bjc.2013.666
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.666
Keywords
This article is cited by
-
Prognostic value of pre-therapeutic nutritional risk factors in elderly patients with locally advanced esophageal squamous cell carcinoma receiving definitive chemoradiotherapy or radiotherapy
BMC Cancer (2023)
-
Incidence, Survival and Prognostic Factors of Oesophagogastric Cancer
Journal of Gastrointestinal Cancer (2022)
-
CT-derived body composition measurements as predictors for neoadjuvant treatment tolerance and survival in gastroesophageal adenocarcinoma
Abdominal Radiology (2022)
-
Management options for post-esophagectomy chylothorax
Surgery Today (2021)
-
Obesity and postoperative outcomes of the patients with laparoscopic adrenalectomy: a systematic review and meta-analysis
BMC Surgery (2020)