Abstract
Background:
The B-cell translocation gene 2 (BTG2) is considered to act as a tumour-suppressor gene because of its antiproliferative and antimigratory activities. Higher levels of BTG2 expression in tumour cells have been linked to a better clinical outcome for several cancer entities. Here, we investigated the expression and function of BTG2 in bladder cancer.
Methods:
The expression of BTG2 in bladder cancer cells was silenced by RNA interference. Cell motility was investigated by wound healing and Boyden chamber assays. The protein expression of BTG2 in bladder cancer was studied by immunohistochemistry.
Results:
We observed that targeted suppression of BTG2 by RNA interference did not result in growth stimulation but led to a substantial inhibition of bladder cancer cell motility. Tissue microarray analyses of bladder cancer cystectomy specimens revealed that higher BTG2 expression levels within the tumours correlated strongly with a decreased cancer-specific survival for bladder cancer patients.
Conclusion:
These results indicate that endogenous BTG2 expression contributes to the migratory potential of bladder cancer cells. Moreover, high levels of BTG2 in bladder cancers are linked to decreased cancer-specific survival. These findings question the conception that BTG2 generally acts as a tumour suppressor and typically represents a favourable clinical marker for cancer patients.
Similar content being viewed by others
Main
The B-cell translocation gene 2 (BTG2) belongs to the APRO (antiproliferative) gene family and is generally considered to exert antioncogenic activities (Matsuda et al, 2001; Duriez et al, 2004; Lim, 2006). Specifically, BTG2 has been shown to inhibit cell proliferation and to induce either G1 or G2 cell cycle arrest, depending on the cellular context (Rouault et al, 1996; Lim et al, 1998; Guardavaccaro et al, 2000; Hong et al, 2005). In addition, BTG2 can counteract ras-induced cellular transformation (Boiko et al, 2006), induce cellular differentiation (el-Ghissassi et al, 2002), and enhance the activity of pro-apoptotic stimuli (Lim et al, 2008). Consistent with a putative tumour-suppressor function, BTG2 expression is downregulated during tumour progression in breast cancers (Kawakubo et al, 2006), gliomas (Calzolari et al, 2008), and prostate cancers (Ficazzola et al, 2001). Moreover, low or absent BTG2 expression levels in tumours have been shown to correlate with a less favourable clinical prognosis for breast and prostate cancer patients (van de Vijver et al, 2002; Mollerstrom et al, 2010; Jalava et al, 2012).
Bladder cancer is a major human cancer with estimated 357 000 new cases diagnosed each year. Approximately 145 000 patients per year will die from the disease (Parkin et al, 2005). Bladder cancer originates in ∼90% from the urothelial (transitional) epithelium of the bladder mucosa (Wu, 2005). Additional histological forms of bladder cancers include squamous cell carcinoma (SCC) that is relatively rare in Western countries, where it constitutes <5% of all bladder cancers (Pons et al, 2011).
Numerous genetic and epigenetic alterations are found in urothelial bladder cancers (Wu, 2005; Mitra and Cote, 2009). Over 50% of cases exhibit defects in p53-linked tumour-suppressor pathways (Wu, 2005). Inactivating somatic alterations of the retinoblastoma tumour-suppressor gene, RB1, are also prevalent in urothelial carcinomas and have been associated with more invasive tumour variants (Cairns et al, 1991). Furthermore, a large proportion of urothelial cancers exhibit aberrant activation of mitogenic signalling pathways linked to the Ras oncoprotein, for example, via somatic ras gene mutations that occur in 30–40% of all urothelial bladder cancers (Wu, 2005) or via missense mutations of the FGFR3 receptor tyrosine kinase gene (Cappellen et al, 1999) that acts as an upstream regulator of HRas (Wu, 2005).
To the best of our knowledge, the status of the BTG2 gene in bladder cancer has not been examined thus far. To address this issue, we (1) analysed BTG2 expression in bladder cancer cells in vitro, (2) tested phenotypic alterations of bladder cancer cells upon silencing of endogenous BTG2 expression by RNA interference (RNAi), (3) investigated BTG2 protein expression in bladder cancer cystectomy specimens by immunohistochemistry, and (4) examined a possible correlation between BTG2 expression levels in the tumours and the clinical prognosis of bladder cancer patients.
Materials and methods
Cells and transfections
All cell lines investigated in this study were authenticated by short tandem repeat profiling or multiplex cell authentication (Castro et al, 2013). All analysed bladder cancer cell lines were derived from urothelial cancers, with the exception of SCaBER cells that were derived from a SCC. Cells were maintained in either RPMI or DMEM (pH 7.2) medium, supplemented with 10% FCS, 50 units ml−1 penicillin, and 50 mg ml−1 streptomycin sulphate. Cells were transfected with synthetic siRNAs (Silencer Select, Life Technologies, Darmstadt, Germany; final concentration of 30 nM) with Dharmafect (Dharmacon, Thermo Fisher Scientific, Lafayette, CO, USA) or with Lipofectamine RNAiMax (Life Technologies). The siRNAs employed in the study targeted the following sequences: siEGFP (5′-GGAGCGCACCAUCUUCUUC-3′), sicontr-1 (5′-CAGUCGCGUUUGCGACUGG-3′) (Hoppe-Seyler et al, 2012), siNeg (5′-UACGACCGGUCUAUCGUAG-3′) (Hoppe-Seyler et al, 2012), siBTG2-3 (5′-GCUCCAUCUGCGUCUUGUA-3′), siBTG2-5 (5′-GAGGCACUCACAGAGCACU-3′), and siBTG2-9 (5′-UGAGCGAGCAGAGGCUUAA-3′). The BTG2-targeting siRNAs bind to non-overlapping domains within the BTG2 mRNA and were applied either alone or in equimolar combination.
All functional analyses (see below) were independently performed at least three times, with consistent results.
Migration assays
Wound healing assays were performed using Culture-Inserts (ibidi, Martinsried, Germany), following the manufacturer’s instructions. Briefly, cells were plated 24 h after transfection onto 24-well plates that contained inserts to generate defined ‘scratch areas’. Inserts were removed after the cells had grown confluent. Cells that had migrated into the scratch areas were visualised after 8–10 h by light microscopy.
Boyden chamber assays were performed using ThinCert- cell culture inserts with a 0.8 μm pore size membrane (Greiner Bio-One, Frickenhausen, Germany), following the manufacturer’s protocol. Briefly, cells were added onto the upper membrane side in the upper chamber 48 h after transfection. Cells that had moved to the lower membrane side were fixed after 24 h and stained with crystal violet.
BrdU assays
Cell proliferation was quantified by measuring BrdU incorporation into the DNA, using the Cell Proliferation ELISA kit (Roche Diagnostics, Mannheim, Germany). Cells were labelled with BrdU for 16 h, harvested 72 h after transfection, and further processed following the manufacturer’s instructions.
Cell cycle analyses
Cells were trypsinised 72 h after transfection, washed in ice-cold phosphate-buffered saline (PBS), and fixed in 80% cold ethanol overnight at −20 °C. Subsequently, cells were pelleted, resuspended in PBS containing 1 mg ml−1 RNAse A (Roche Diagnostics) and 25 mg ml−1 propidium iodide (Sigma-Aldrich, München, Germany), and incubated for 30 min at RT. Cell cycle analyses were performed using FACSCalibur (BD Biosciences, Heidelberg, Germany) with CellQuest Pro software. Quantitation of the percentage of cells in individual cell cycle phases was performed using FlowJo software (Tree Star, Ashland, OR, USA), applying the Dean-Jett-Fox model (Fox, 1980).
RNA extraction, quantitative real-time reverse transcription–PCR (qRT–PCR), and immunoblot analyses
The RNA was purified using PureLink RNA Mini Kit (Life Technologies). The RNA concentrations were measured with NanoDrop ND-1000 (Thermo Fisher Scientific, Wilmington, DE, USA), at 260 nm. Reverse transcription of 1 μg RNA was performed using the oligo-dT primer and ProtoScript M-MuLV Taq RT–PCR Kit (New England Biolabs, Frankfurt, Germany). Expression levels were determined by real-time PCR with a 7300 Real-Time PCR System detector (Life Technologies), using the SYBR green PCR Master Mix (Life Technologies), supplemented to 500 nM of each forward and reverse primer. The BTG2 (NM_006763.2) expression was determined using forward primer 5′-CTCACCTGCAAGAACCAAGTG-3′ and reverse primer 5′- AGTTCCCCAGGTTGAGGTATGT-3′. The GAPDH primer sequences and cycling conditions have been previously described (Fussbroich et al, 2011). The sizes of the PCR products were initially verified by agarose gel electrophoresis and subsequently checked by melting point analysis after each reaction. Relative quantification was performed using the comparative Ct (2−ΔΔCt) method (Livak and Schmittgen, 2001). Data are presented as the fold difference in gene expression normalised to GAPDH and relative to a calibrator sample. Statistical significance of differences in measured variables between controls and treated groups was determined by paired t-test using the Sigma Plot software (Systat Software Inc., San Jose, CA, USA). Differences were considered significant at P<0.05.
For immunoblot analyses, total protein extracts were prepared 72 h after transfection, as described previously (Hoppe-Seyler et al, 2012). Briefly, 30 μg of protein extract was separated by 12.5% SDS–PAGE, transferred to an Immobilon-P membrane (Millipore, Bedford, MA, USA), and analysed by enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK) using a rabbit polyclonal anti-BTG2 antibody (GenWay Biotech, San Diego, CA, USA) or an anti-α-tubulin antibody (CP06, Calbiochem, Darmstadt, Germany).
Patients
Clinical data of patients (n=273) who underwent radical cystectomy between 1998 and 2009 for muscle-invasive and refractory nonmuscle-invasive urinary bladder cancer (histopathological subtypes: urothelial carcinoma, SCC, and others) at three centres (Departments of Urology, Universities of Heidelberg and Bonn, Germany, and University of Mansoura, Egypt) were entered into a tumour database. Tumour stage was classified according to the tumour node metastasis staging system of 2002 (Greene et al, 2002) Patients were prospectively evaluated every 3 months for the first 2 years after surgery, every 6 months for the next 3 years, and yearly thereafter (chest X-ray or thoracic CT scan; abdominal/pelvic sonography or CT scan or MRI; X-ray intravenous urography or CT urography or MRI urography; urine cytology; serum chemistry). Chemotherapy regimens consisted of methotrexate, vinblastine, epirubicin, cisplatin, and gemcitabine. Of the 273 patients, 5 (1.8%) received neoadjuvant chemotherapy, 43 (15.8%) patients underwent adjuvant chemotherapy, and 2 (0.7%) patients underwent adjuvant radiation therapy for positive lymph node status and/or extravesical extension. The work was covered by a votum of the ethical committee of the University of Heidelberg No. 206/2005. Informed and/or written consent was obtained from each patient. Clinical and pathological features of patients scored for BTG2 expression (n=183; urothelial carcinoma of the bladder n=127, SCC of the bladder n=46, other histopathological subtypes n=10) after exclusion of insufficient tumour tissue or fixation artefacts are summarised in Supplementary Table S1.
Tissue microarrays
A tissue microarray (TMA) containing 273 primary tumour tissue samples was created by the tissue bank of the National Center for Tumor Diseases (NCT) Heidelberg. Representative tissue blocks were selected as donor blocks for the TMA. Sections were cut from each donor block and stained with haematoxylin and eosin. Then, a morphologically representative region was chosen from each of the bladder cancers. At least one cylindrical core tissue specimen per tumour block (diameter, 0.6 mm) was punched from these regions and arrayed into the recipient paraffin block using a semiautomatic system (Beecher Instruments, Silver Spring, MD, USA).
Immunohistochemistry
Tissue microarray slides were dewaxed and rehydrated using xylene and a series of graded alcohols, followed by heat-induced antigen retrieval using a target retrieval solution (S2031, DakoCytomation, Glostrup, Denmark) in a pressure cooker for 10 min. Immunohistochemical staining was performed on an automated staining system (Techmate 500, DakoCytomation) with a 1 : 200 dilution of an anti-BTG2 antibody (GenWay Biotech) (Mollerstrom et al, 2010) for 30 min. An avidin-biotin-complex peroxidase technique using aminoethylcarbazole for visualisation and haematoxylin for counterstaining was applied. We used the following materials: ChemMate Detection Kit (K5003, DakoCytomation), ChemMate Buffer Kit (K5006, DakoCytomation), and, for reduction of nonspecific avidin/biotin-related staining, the Avidin/Biotin Blocking Kit (SP-2001, Vector Laboratories, Burlingame, CA, USA). Sections were thoroughly washed, glass covered, and analysed by light microscopy (Olympus Vanox-T, Hamburg, Germany) using a magnification of up to × 400. As negative control for the immunohistochemical staining procedure, the primary antibody was omitted, with all other experimental conditions kept constant.
For immunohistochemical assessment of BTG2 expression, the product of the scores of staining frequency and intensity of immunoreactive tumour cells was calculated on the following scoring system: the frequency ranged from 0% to 100% of BTG2-positive tumour cells, and the intensity comprised 0=no to 3=high. The final immunohistochemical score (ranging from 0 to 300) is obtained by multiplication of the intensity score and the frequency score. A cutoff between low, moderate, and high expression was set as follows: low BTG2 expression, ⩽20; moderate BTG2 expression, >20–160; and high BTG2 expression, >160–300. These immunohistochemical scores were determined using the sample quartiles (Q1=lower quartile, Q2=median, and Q3=upper quartile) as cut-points for categorisation of the continuous variable BTG2 expression. The arrays were independently scored by two researchers (NW and GH) blinded for patient outcomes. For the few instances of discrepant scoring, a consensus score was determined.
Study design
A retrospective study design was chosen. The cancer-specific survival of patients was calculated from the date of bladder surgery. The survival end point was the date of last follow-up or date of death because of cancer. Kaplan–Meier estimates were used to describe survival rates including pointwise asymptotic 95% confidence intervals using Greenwood’s formula for standard error. Patients with proven tumour-independent death were censored. The following clinical and pathological features were studied for their prognostic relevance on long-term survival of urinary bladder cancer patients: age (<65 years vs ⩾65 years), sex (male vs female), tumour stage (T2, T3, T4 vs T1), lymph node involvement (negative vs positive), metastases (M0 vs M1), grading (grade 2, 3/4 vs 1), histopathological subtype (urothelial carcinoma vs SCC vs other types), concomitant carcinoma in situ (negative vs positive), lymphovascular invasion (negative vs positive), and cytoplasmic/membranous BTG2 expression (moderate, high vs low).
Statistical analysis
Association between important prognostic factors and BTG2 levels was evaluated by Fisher’s exact test. For the evaluation of prognostic factors, the study population was left as a whole cohort of patients (all histologies) or divided into subgroups of urothelial carcinoma and SCC of the bladder. No data-driven combination of adjacent categories related to BTG2 expression was carried out to retain the confirmatory nature of the evaluation of BTG2. Univariate and multivariate analyses of prognostic factors were carried out within the Cox proportional hazards model using complete case analysis. For each prognostic factor the hazard ratio in the univariate analysis and the adjusted hazard ratio in the multivariate analysis are given, including the 95% confidence interval. A P-value of <0.05 was considered significant. For further description of the predictive value of BTG2, the concordance probability (Goenen and Heller, 2005) of the Cox model including BTG2 were calculated and compared with the model excluding BTG2 but retaining all other variables. Additionally, a multivariate analysis with the BTG2 expression as continuous predictor and the clinicopathological characteristics as categorical predictors was performed, using the multivariate fractional polynomial (MFP) approach (Royston et al, 1999). With this method, it was possible to keep the BTG2 predictor as a continuous variable. The statistical analysis system SAS, Version 9.3 for Windows (SAS Institute Inc., Cary, NC, USA) was used for these analyses. For the calculation of the concordance probability and the associated standard errors, the SAS Code offered by Goenen and Heller (2005) was used.
Results
BTG2 expression in bladder cancer cell lines
In order to analyse the expression of the BTG2 gene in bladder cancer cells in vitro, we investigated a panel of five tumour-derived bladder cancer cell lines by qRT–PCR. All tested cell lines expressed detectable levels of BTG2 mRNA, showing differences in relative amounts ranging up to approximately three levels of magnitude (Figure 1A). The expression of BTG2 has been reported to be enhanced with increasing cell density in renal cell carcinoma cells (Struckmann et al, 2004). In line with this finding, SCaBER bladder cancer cells also exhibited a significant induction of BTG2 mRNA concentrations (3.4-fold) at confluency when compared with BTG2 expression levels under semiconfluent conditions (Figure 1A). In contrast, however, RT4, RT112, T24, and 5637 cells did not show significant alterations of BTG2 mRNA amounts when compared at semiconfluent or confluent conditions (Figure 1A).
The expression of BTG2 in bladder cancer cells and its modulation by RNA interference. (A) Quantitative real-time reverse transcription–PCR (qRT–PCR) analyses of BTG2 mRNA expression. Data are represented as fold differences in gene expression, normalised to GAPDH expression. White columns, semiconfluent conditions; grey columns, confluent conditions. Standard deviations from at least two independent experiments are indicated. *Statistically significant difference (P<0.05). (B) The qRT–PCR analyses of BTG2 mRNA levels following treatment with 0.5 μg ml−1 doxorubicin for 24 h. The mRNA levels of BTG2 in doxorubicin-treated cells are indicated relative to those in corresponding solvent-treated control cells (arbitrarily set at 1.0). Standard deviations are indicated. (C) The qRT–PCR analyses of BTG2 mRNA levels following treatment with 1 μ M retinoic acid (r.a.) for 24 h. The mRNA levels of BTG2 in r.a.-treated cells are indicated relative to those in corresponding solvent-treated control cells (arbitrarily set at 1.0). Standard deviations are indicated. (D) The qRT–PCR analyses of BTG2 mRNA levels following siRNA treatment of bladder cancer cells. The siBTG2-3, siBTG2-5, and siBTG2-9 target three different regions within the BTG2 mRNA; siBTG2-5/9 corresponds to a pool of equal amounts of siBTG2-5 and siBTG2-9; siNeg and sicontr-1 served as negative control siRNAs. For each cell line, BTG2 mRNA levels are indicated relative to those in corresponding siNeg-treated control cells (arbitrarily set at 1.0 for each cell line). Standard deviations are indicated. (E) Immunoblot analyses of BTG2 protein levels upon siRNA treatment of RT112 bladder cancer cells. Mock, cells undergoing the transfection procedure in the absence of siRNAs; siEGFP and sicontr-1 served as negative control siRNAs; siBTG2-3 and siBTG2-5 target two different regions in the BTG2 mRNA; siBTG2-3/5 corresponds to a pool of equal amounts of siBTG2-3 and siBTG2-5. Tubulin, loading control.
Next, we tested whether BTG2 expression in bladder cancer cells can be modulated by agents that have been reported to affect BTG2 levels in other cell types. The BTG2 gene is transcriptionally activated by p53 that can mediate BTG2 induction by genotoxic agents, like doxorubicin (Rouault et al, 1996; Elmore et al, 2005; Lim et al, 2008) or ionising radiation (Rouault et al, 1996). As previously reported (Rouault et al, 1996), doxorubicin treatment led to an increase of BTG2 mRNA levels in MCF-7 breast cancer cells that served as a positive control for our experiments (Figure 1B). A comparably high induction of BTG2 expression was observed for T24 bladder cancer cells. In contrast, however, BTG2 mRNA levels were only marginally increased, if at all, in RT4, RT112, SCaBER, and 5637 bladder cancer cells (Figure 1B).
In addition, BTG2 mRNA levels can be enhanced in MCF-7 cells by retinoic acid (r.a.) treatment, suggesting that r.a. may act antiproliferative in these cells via BTG2 stimulation (Donato et al, 2007). In accordance with this study, we also observed a clear upregulation of BTG2 mRNA levels in MCF-7 cells following r.a. treatment (Figure 1C). In contrast, however, none of the tested bladder cancer cell lines (RT4, RT112, SCaBER, and T24) exhibited a more than marginal increase in BTG2 expression, if at all, indicating that r.a.-mediated BTG2 induction is largely abolished in these cells (Figure 1C).
For RNAi analyses, we generated synthetic siRNAs that target different regions of the BTG2 transcript. Figure 1D shows that these siRNAs substantially reduced BTG2 mRNA concentrations, indicating that BTG2 is amenable to RNAi-mediated suppression in bladder cancer cells. The inhibitory effects of RNAi was also observed at the protein level, as shown for RT112 cells (Figure 1E). For subsequent functional investigations, we concentrated on RT112, T24, SCaBER, and 5637 bladder cancer cells as these cells could be efficiently transfected and exhibited a substantial downregulation of endogenous BTG2 expression by RNAi (Figure 1D).
BTG2 silencing blocks the migration capacity of bladder cancer cells
In order to assess the phenotypic effects of BTG2 suppression in bladder cancer cells, we tested their proliferative activity by analysing BrdU incorporation into the cellular DNA. Under experimental conditions where a substantial reduction of endogenous BTG2 expression is achieved by RNAi (Figure 1D), we did not observe increased cellular proliferation for any of the tested bladder cancer cell lines (Figure 2A). Rather, BTG2 inhibition led to a slight decrease in cellular proliferation. Thus, our data indicate that suppression of endogenous BTG2 expression does not enhance cell proliferation of bladder cancer cell lines. In line with this interpretation, we also did not observe appreciable alterations in cell cycle distributions of bladder cancer cells upon inhibition of BTG2 expression (Figure 2B).
Analysis of bladder cancer cell proliferation upon RNAi-mediated inhibition of BTG2 expression. (A) Measurement of BrdU incorporation in RT112, SCaBER, 5637, and T24 bladder cancer cells. For each cell line, the percentage of BrdU incorporation upon siRNA application is indicated relative to that in corresponding siNeg-treated control cells (set at 100% for each cell line). The siNeg and sicontr-1 served as control siRNAs; siBTG2-5/9 corresponds to a pool of equal amounts of siBTG2-5 and siBTG2-9. Standard deviations are indicated. (B) Fluorescence-activated cell sorting (FACS) analyses investigating the cell cycle distribution of bladder cancer cells following inhibition of BTG2 expression. Percentages of cells in the G1, S, or G2/M phases upon treatment with control siRNAs (siNeg, sicontr-1) or with pooled siRNAs blocking BTG2 expression (siBTG2-5/9) are indicated.
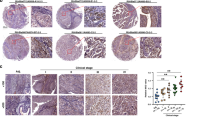
The suppression of BTG2 by RNAi has been linked to increased cell migration in breast cancer cells (Takahashi et al, 2011). In order to investigate whether BTG2 may contribute to the regulation of this process in bladder cancer cells, BTG2 expression was repressed by RNAi in RT112, T24, SCaBER, and 5637 cells. Subsequently, the migration potential of the cells was compared with control-transfected cells in ‘wound healing (scratch)’ assays (Liang et al, 2007). Contrary to the findings reported for breast cancer cells, we observed a substantially decreased migration rate for all four investigated bladder cancer cell lines upon suppression of endogenous BTG2 expression. A representative assay is shown for 5637 cells in Figure 3A, and a compilation of the results for all four tested bladder cancer cell lines is presented in Figure 3B.
‘Wound healing (scratch)’ assays analysing the effects of BTG2 suppression on the migration of bladder cancer cells. (A) The 5637 cells were transfected with the indicated siRNAs and subjected to in vitro wound healing assays. Images were captured by light microscopy. Dotted lines define the areas lacking cells (‘scratches’), at time point 0. Cells that had migrated into the scratch areas were counted after 8–10 h. The siNeg and sicontr-1 served as control siRNAs; siBTG2-5 and siBTG2-9 are two unrelated siRNAs blocking BTG2 expression. (B) Quantification of the effect of BTG2 suppression on the migration of RT122, SCaBER, 5637, and T24 cells in wound healing assays. For each cell line, percentages of migrated cells were determined relative to cells treated with control siRNA siNeg (set at 100%). Standard deviations are indicated.
To investigate this issue by an independent experimental method, we performed ‘Boyden chamber’-based motility assays (‘filter membrane migration assays’). In contrast to the monolayer wound healing assay, the Boyden chamber assay is a two-compartment assay that requires cells to release intercellular contacts in order to migrate through a porous membrane (Chen, 2005). Again, all four tested bladder cancer cell lines exhibited a substantially decreased migratory activity upon suppression of BTG2 expression (Figure 4).
‘Boyden chamber’ assays analysing the effects of BTG2 suppression on the migration of bladder cancer cells. Cells were sown on the upper side of a porous membrane separating the two compartments of the migration chamber. Cells that had migrated through the pores to the lower side of the membrane were fixed and stained with crystal violet. Individual bladder cancer cell lines are indicated. n.d., not done. siNeg, sicontr-1 served as negative control siRNAs; siBTG2-5 and siBTG2-9 are two unrelated siRNAs blocking BTG2 expression; siBTG2-5/9 corresponds to an equimolar mixture of siBTG2-5 and siBTG2-9.
Taken together, these results show that silencing of endogenous BTG2 expression markedly reduces the motility of bladder cancer cells and thus indicate that BTG2 substantially contributes to their migratory activity.
Differential BTG2 expression in distinct histologies of bladder cancer
In order to investigate BTG2 expression in urinary bladder cancer in vivo, a multi-institutional TMA was constructed. Expression of BTG2 was analysed by immunohistochemistry. Altogether, 183 cases were scored for BTG2 expression. Cases with insufficient tumour tissue or fixation artefacts (n=90) were excluded from further analyses.
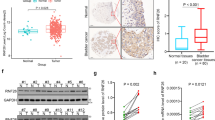
Typical examples of BTG2 protein expression in urinary bladder cancers are depicted in Figure 5. In the cytoplasm and/or at the cell membrane of bladder cancer cells (Figure 5A and B), BTG2 was detected, as has been previously reported for the BTG2 expression pattern in breast cancer cells (Mollerstrom et al, 2010). Notably, the amounts of BTG2 expression differed between the distinct bladder cancer histologies. In urothelial carcinomas, 75 samples (59.0%) exhibited high (immunohistochemical score: >160–300) BTG2 levels (Figure 5A), 41 cases (32.3%) moderate (>20–160) BTG2 levels (Figure 5B), and only 11 cases (8.7%) low (⩽20) BTG2 levels (Figure 5C). In contrast, the large majority of SCCs (37 out of 46 samples=80.4%) displayed only low (⩽20) BTG2 levels, with most samples exhibiting undetectable BTG2 expression (Figure 5D). Only six SCC samples displayed moderate (>20–160) BTG2 levels and three SCC samples exhibited high (>160–300) BTG2 levels.
The expression of BTG2 in urinary bladder cancer. Overviews (left panels) and higher-resolution presentations (right panels) of the boxed areas. (A) Urothelial carcinoma of the urinary bladder, showing high cytoplasmic/membranous BTG2 expression (indicated by arrows). (B) Urothelial carcinoma of the urinary bladder, showing moderate cytoplasmic/membranous BTG2 expression (indicated by arrows). (C) Urothelial carcinoma of the urinary bladder, showing low cytoplasmic/membranous BTG2 expression. (D) Squamous cell carcinoma of the urinary bladder, showing no BTG2 expression. Scale bars, 5 μm.
BTG2 expression correlates strongly with cancer-specific survival in urinary bladder cancer
Next, the survival of patients with urinary bladder cancer was calculated from the time of bladder surgery. Median follow-up of patients still alive (n=95) was 6.7 years (range 0.1–11.7 years). Until July 2010, 63 patients (34.4%) had died of their disease. The cancer-specific survival rate at 1 year and at 5 years after surgery for the whole cohort of patients was 82.6% (95 CI 76.2–87.4%) and 64.9% (95 CI 57.3–71.5%), respectively. The clinical and pathological features of patients are summarised in Supplementary Table S1.
Patients with one of the following criteria (age⩾65 years, advanced tumour stages (T3 and T4), high grading (grade 3/4), no lymphovascular invasion, and urothelial carcinoma) were found to have significantly more often higher BTG2 levels when compared with patients with age <65 years, tumour stages 1 and 2, low grading (grades 1 and 2), and SCC (Supplementary Table S2).
As shown in Figure 6A, Kaplan–Meier cancer-specific survival curves of the whole cohort of patients (all histologies) revealed a significantly worse prognosis in patients with high BTG2 levels compared with low and moderate BTG2 levels (P=0.02). In subgroup analyses of patients with urothelial carcinoma or SCC of the bladder, high levels of BTG2 showed a strong tendency towards being an independent predictor of poor cancer-specific survival (P=0.05 and P=0.12, respectively; Figure 6B and C).
On univariate survival analyses, the risk of death from bladder cancer for patients showing high BTG2 levels in the tumour was significantly enhanced above that for bladder cancer patients who exhibited low BTG2 levels (Supplementary Table S3, HR 2.01, P=0.007). Apart from BTG2, the following clinical and histopathological features showed a statistically significant impact on cancer-specific survival in bladder cancer patients in univariate analyses: tumour stage, grading, histopathological subtype, lymph node involvement, metastatic disease, age, and lymphovascular invasion (Supplementary Table S3). In subgroup analyses of urothelial carcinoma, the risk of death for patients with high BTG2 levels in the tumour was also clearly enhanced above that for patients who have low BTG2 levels (Supplementary Table S4, HR=2.4, P=0.02). In contrast, no significant association between BTG2 and the risk of death was obtained in subgroup analyses of SCC (HR=3.14, P=0.14).
Next, we investigated whether BTG2 may also independently correlate with cancer-specific survival in urinary bladder cancer. Multivariate Cox regression analyses on bladder cancer outcome included tumour stage, lymph node involvement, metastatic disease, grading, lymphovascular invasion, concomitant carcinoma in situ, age, sex, histopathological subtype, and BTG2 expression. These analyses revealed that high BTG2 expression displays a strong tendency to be an independent prognostic marker of cancer-specific survival (Supplementary Table S3, HR=2.31, P=0.09). Apart from BTG2 expression, histopathological subtype, lymph node involvement, and grading were selected as important prognostic indicators (Supplementary Table S3). Furthermore, in a multivariate analysis excluding BTG2, the same variables without lymph node involvement were selected as prognostic indicators (Supplementary Table S3).
In a prognostic model based on the multivariate fractional polynomial (MFP) method, BTG2 as continuous variable and the same categorical variables as in the multivariate Cox regression model were included in the analysis. The MFP algorithm works iteratively, selecting out covariates with a P-value >0.15. At the final cycle, the MFP algorithm selected the following predictors as important prognostic indicators on cancer-specific survival: BTG2, histopathological subtype, tumour stage, lymph node involvement, and grading (Supplementary Table S5).
Finally, for further evaluation of the predictive value of BTG2 expression, the concordance probability of the Cox regression models including or excluding BTG2 was calculated. The concordance probability of the Cox regression models including the BTG2 status was 0.743 with a standard error of 0.027, compared with 0.737 with a standard error of 0.028 in models excluding the BTG2 status but retaining all other variables.
Discussion
The present study is the first investigation of the status and function of the putative tumour-suppressor gene BTG2 in cancers of the urinary bladder. We obtained two major unexpected results. First, we observed that BTG2 suppression resulted in a strongly reduced cell motility. These findings indicate that BTG2 expression in bladder cancer cells contributes to their migration ability. Second, and in contrast to reports from other cancer entities, we observed that elevated BTG2 expression levels in bladder cancers were linked to a strongly decreased cancer-specific survival.
Our conclusion that BTG2 suppression leads to a reduced migration capacity of bladder cancer cells is based on different BTG2-targeting siRNAs that yielded confirmatory results and on two independent cell migration assays: the wound healing (scratch) and the Boyden chamber assay. These two tests methodologically differ in that the Boyden chamber assay requires cells to release intercellular contacts and therefore allows cell motility analyses that are largely independent of cell–cell interactions (Chen, 2005). Both assays yielded consistent results, showing a profound reduction of bladder cancer cell migration upon BTG2 suppression. The observation that BTG2 suppression did not markedly affect cell viability or cell proliferation of bladder cancer cells, as assessed by BrdU and cell cycle analyses, provides strong evidence that the results of the migration assays reflect real cell migration and not altered cell proliferation.
The positive correlation between BTG2 expression and the migration capacity of bladder cancer cells contrasts the situation in breast cancer cells where these parameters exhibited an inverse correlation (Takahashi et al, 2011). This indicates that BTG2 can exert differential effects on cellular migration, depending on the cellular context. The issue of cancer cell migration is of particular interest for the genesis of urothelial bladder cancers that often present themselves as multifocal tumours in the urothelial tract of a single patient. Accumulating molecular evidence suggests that these lesions are descendants of the intraepithelial (or intraluminal) spread of a single transformed cell (Habuchi, 2005; Wu, 2005).
Interesting differences were also observed for the in vivo BTG2 expression pattern between urothelial and SCC of the bladder. Less than 10% of the urothelial carcinomas exhibited low or undetectable BTG2 levels – in contrast to ∼80% of SCCs. This observation is likely to reflect differences in the molecular pathogenesis of these two histological bladder cancer variants (Wu, 2005; Pons et al, 2011). In addition, we observed survival differences by the two histological subtypes: although the grading of urothelial carcinoma was significantly worse than in SCC (Supplementary Table S1), it was more likely to die because of a SCC than of an urothelial carcinoma of the bladder (Supplementary Table S3, HR 2.48).
Previous studies showed that higher BTG2 expression levels in tumours were linked to a better clinical prognosis for breast, renal, and prostate cancer patients (Struckmann et al, 2004; Mollerstrom et al, 2010; Jalava et al, 2012). In contrast, we observed a strongly increased risk of death from urinary bladder cancer for patients exhibiting high BTG2 levels in the tumour tissue when compared with patients with low BTG2 levels (Figure 6A). This increased risk was statistically significant in univariate Cox regression analyses (Supplementary Table S3, P=0.007) and remained as a strong tendency in multivariate Cox regression analyses (Supplementary Table S3, P=0.09). In multivariate fractional polynomials, (Supplementary Table S5, P=0.014), an alternative approach using BTG2 as a continuous variable, BTG2 was also found to be an independent prognostic factor. In subgroup analyses of urothelial carcinoma, univariate Cox regression analyses again demonstrated increased levels of BTG2 as a significant risk factor for cancer-specific death (Supplementary Table S4, P=0.02). In contrast, in subgroup analyses of SCC, this significance was lost (Supplementary Table S4, P=0.14), possibly because of the small number of patients (n=46). The concordance probability estimated for the multivariate Cox regression model incorporating BTG2 was 0.743 with a standard error of 0.027. The estimation of the concordance probability after excluding BTG2 was in a very similar range (0.737, standard error of 0.028). Taken together, and in marked contrast to the results for other tumour entities, these findings provide evidence that higher levels of BTG2 expression within urinary bladder cancers are associated with a strongly decreased cancer-specific survival of affected patients. Despite this evidence, we did not see an increase in the concordance probability including or excluding BTG2 expression in our predictive model. To increase the predictive accuracy, BTG2 expression should be included into molecular multimarker panels as these are more likely to accurately predict outcomes in invasive bladder cancer than any single marker (Gakis et al, 2012).
In conclusion, our study shows that inhibition of endogenous BTG2 expression substantially reduced the motility of bladder cancer cells. This finding indicates that the endogenous BTG2 expression in bladder cancer cells contributes to their migratory potential and thereby may promote tumourigenesis. Furthermore, we found that increased intratumoural BTG2 levels are linked to a less favourable clinical outcome for bladder cancer patients. These results are in marked contrast to previous investigations in other cancer entities where BTG2 is thought to act antioncogenic (e.g., antiproliferative, antimigratory) and where increased BTG2 expression is linked to a more favourable clinical outcome. Our findings thus indicate that the endogenous activities of BTG2 are cell type dependent. They question the conception that BTG2 generally acts as a tumour suppressor and that BTG2 expression typically represents a favourable clinical marker in cancer patients.
Change history
05 March 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Boiko AD, Porteous S, Razorenova OV, Krivokrysenko VI, Williams BR, Gudkov AV (2006) A systematic search for downstream mediators of tumor suppressor function of p53 reveals a major role of BTG2 in suppression of Ras-induced transformation. Genes Dev 20 (2): 236–252
Cairns P, Proctor AJ, Knowles MA (1991) Loss of heterozygosity at the RB locus is frequent and correlates with muscle invasion in bladder carcinoma. Oncogene 6 (12): 2305–2309
Calzolari F, Appolloni I, Tutucci E, Caviglia S, Terrile M, Corte G, Malatesta P (2008) Tumor progression and oncogene addiction in a PDGF-B-induced model of gliomagenesis. Neoplasia 10 (12): 1373–1382, following 1382
Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, Chopin D, Thiery JP, Radvanyi F (1999) Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet 23 (1): 18–20
Castro F, Dirks WG, Fahnrich S, Hotz-Wagenblatt A, Pawlita M, Schmitt M (2013) High-throughput SNP-based authentication of human cell lines. Int J Cancer 132 (2): 308–314
Chen HC (2005) Boyden chamber assay. Methods Mol Biol 294: 15–22
Donato LJ, Suh JH, Noy N (2007) Suppression of mammary carcinoma cell growth by retinoic acid: the cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling. Cancer Res 67 (2): 609–615
Duriez C, Moyret-Lalle C, Falette N, El-Ghissassi F, Puisieux A (2004) BTG2, its family and its tutor. Bull Cancer 91 (7-8): E242–E253
el-Ghissassi F, Valsesia-Wittmann S, Falette N, Duriez C, Walden PD, Puisieux A (2002) BTG2(TIS21/PC3) induces neuronal differentiation and prevents apoptosis of terminally differentiated PC12 cells. Oncogene 21 (44): 6772–6778
Elmore LW, Di X, Dumur C, Holt SE, Gewirtz DA (2005) Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: implications for treatment response. Clin Cancer Res 11 (7): 2637–2643
Ficazzola MA, Fraiman M, Gitlin J, Woo K, Melamed J, Rubin MA, Walden PD (2001) Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis 22 (8): 1271–1279
Fox MH (1980) A model for the computer analysis of synchronous DNA distributions obtained by flow cytometry. Cytometry 1 (1): 71–77
Fussbroich B, Wagener N, Macher-Goeppinger S, Benner A, Falth M, Sultmann H, Holzer A, Hoppe-Seyler K, Hoppe-Seyler F (2011) EZH2 depletion blocks the proliferation of colon cancer cells. PloS One 6 (7): e21651
Gakis G, Schwentner C, Todenhöfer T, Stenzl A (2012) Current status of molecular markers for prognostification and outcome in invasive bladder cancer. BJU Int 110 (2): 233–237
Goenen M, Heller G (2005) Concordance probability and discriminatory power in proportional hazards regression. Biometrika 92 (4): 965–970
Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M (2002) AJCC Cancer Staging Manual. Springer: Berlin
Guardavaccaro D, Corrente G, Covone F, Micheli L, D’Agnano I, Starace G, Caruso M, Tirone F (2000) Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol Cell Biol 20 (5): 1797–1815
Habuchi T (2005) Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int J Urol 12 (8): 709–716
Hong JW, Ryu MS, Lim IK (2005) Phosphorylation of serine 147 of tis21/BTG2/pc3 by p-Erk1/2 induces Pin-1 binding in cytoplasm and cell death. J Biol Chem 280 (22): 21256–21263
Hoppe-Seyler K, Sauer P, Lohrey C, Hoppe-Seyler F (2012) The inhibitors of nucleotide biosynthesis leflunomide, FK778, and mycophenolic acid activate hepatitis B virus replication in vitro. Hepatology 56 (1): 9–16
Jalava SE, Urbanucci A, Latonen L, Waltering KK, Sahu B, Janne OA, Seppala J, Lahdesmaki H, Tammela TL, Visakorpi T (2012) Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene 31 41: 4460–4471
Kawakubo H, Brachtel E, Hayashida T, Yeo G, Kish J, Muzikansky A, Walden PD, Maheswaran S (2006) Loss of B-cell translocation gene-2 in estrogen receptor-positive breast carcinoma is associated with tumor grade and overexpression of cyclin d1 protein. Cancer Res 66 (14): 7075–7082
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2 (2): 329–333
Lim IK (2006) TIS21 (/BTG2/PC3) as a link between ageing and cancer: cell cycle regulator and endogenous cell death molecule. J Cancer Res Clin Oncol 132 (7): 417–426
Lim IK, Lee MS, Ryu MS, Park TJ, Fujiki H, Eguchi H, Paik WK (1998) Induction of growth inhibition of 293 cells by downregulation of the cyclin E and cyclin-dependent kinase 4 proteins due to overexpression of TIS21. Mol Carcinog 23 (1): 25–35
Lim YB, Park TJ, Lim IK (2008) B cell translocation gene 2 enhances susceptibility of HeLa cells to doxorubicin-induced oxidative damage. J Biol Chem 283 (48): 33110–33118
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 (4): 402–408
Matsuda S, Rouault J, Magaud J, Berthet C (2001) In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett 497 (2-3): 67–72
Mitra AP, Cote RJ (2009) Molecular pathogenesis and diagnostics of bladder cancer. Annu Rev Pathol 4: 251–285
Mollerstrom E, Kovacs A, Lovgren K, Nemes S, Delle U, Danielsson A, Parris T, Brennan DJ, Jirstrom K, Karlsson P, Helou K (2010) Up-regulation of cell cycle arrest protein BTG2 correlates with increased overall survival in breast cancer, as detected by immunohistochemistry using tissue microarray. BMC Cancer 10: 296
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55 (2): 74–108
Pons F, Orsola A, Morote J, Bellmunt J (2011) Variant forms of bladder cancer: basic considerations on treatment approaches. Curr Oncol Rep 13 (3): 216–221
Rouault JP, Falette N, Guehenneux F, Guillot C, Rimokh R, Wang Q, Berthet C, Moyret-Lalle C, Savatier P, Pain B, Shaw P, Berger R, Samarut J, Magaud JP, Ozturk M, Samarut C, Puisieux A (1996) Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat Genet 14 (4): 482–486
Royston P, Ambler G, Sauerbrei W (1999) The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 28 (5): 964–974
Struckmann K, Schraml P, Simon R, Elmenhorst K, Mirlacher M, Kononen J, Moch H (2004) Impaired expression of the cell cycle regulator BTG2 is common in clear cell renal cell carcinoma. Cancer Res 64 (5): 1632–1638
Takahashi F, Chiba N, Tajima K, Hayashida T, Shimada T, Takahashi M, Moriyama H, Brachtel E, Edelman EJ, Ramaswamy S, Maheswaran S (2011) Breast tumor progression induced by loss of BTG2 expression is inhibited by targeted therapy with the ErbB/HER inhibitor lapatinib. Oncogene 30 (27): 3084–3095
van de Vijver MJ, He YD, van‘t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347 (25): 1999–2009
Wu XR (2005) Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer 5 (9): 713–725
Acknowledgements
We thank Barbara Schreiber and Andrea Hain (NCT Tissue Bank, Heidelberg) for expert technical assistance. We are grateful to Hildegard Jakobi (prospective tumor database, Department of Urology, University of Heidelberg), Karl-Heinz Ellsässer (Centre for Information and Medical Technology, University of Heidelberg), and Biljana Gigic (Data Management, NCT Clinical Trial Center, German Cancer Research Center, Heidelberg) for excellent help with the patient data. We thank the National Center for Tumor Diseases (NCT), Heidelberg, for creating the TMA. We thank Hans Krause (Department of Urology, Charité, Berlin) for the gift of SCaBER cells. This study was supported by a grant from the Else-Kröner-Fresenius-Stiftung to KH-S (P10/09//A139/08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wagener, N., Bulkescher, J., Macher-Goeppinger, S. et al. Endogenous BTG2 expression stimulates migration of bladder cancer cells and correlates with poor clinical prognosis for bladder cancer patients. Br J Cancer 108, 973–982 (2013). https://doi.org/10.1038/bjc.2012.573
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.573
Keywords
This article is cited by
-
Mir-25 Promotes Metastasis of Esophageal Cancer by Targeting BTG2
Applied Biochemistry and Biotechnology (2023)
-
Identification of Novel Diagnostic Biomarkers and Classification Patterns for Osteoarthritis by Analyzing a Specific Set of Genes Related to Inflammation
Inflammation (2023)
-
Unraveling the role of microRNA/isomiR network in multiple primary melanoma pathogenesis
Cell Death & Disease (2021)
-
Up-regulated LINC01234 promotes non-small-cell lung cancer cell metastasis by activating VAV3 and repressing BTG2 expression
Journal of Hematology & Oncology (2020)
-
A fail-safe system to prevent oncogenesis by senescence is targeted by SV40 small T antigen
Oncogene (2020)