Abstract
Background:
Regulatory T cells (Tregs) are commonly identified by expression of the transcription factor FOXP3 and are conventionally thought to promote cancer progression by suppressing anti-tumour immune responses. We examined the relationship between FOXP3+ tumour-infiltrating lymphocytes (TIL) and prognosis in oestrogen receptor (ER)-negative breast cancer, a tumour subtype with poor clinical outcome in which TIL are abundant.
Methods:
FOXP3+ and CD8+ TIL were assessed by immunohistochemistry in a cohort of 175 ER– breast tumours. Results were confirmed in an independent data set of 78 ER– breast tumours with publically available gene expression data.
Results:
High FOXP3+ TIL levels were strongly associated with prolonged recurrence-free survival (HR=0.461, P=0.0002), particularly among basal-like tumours (HR=0.280, P=0.0001), for which FOXP3 status was independent of standard prognostic factors. Over 75% of FOXP3+ TIL in triple negative breast tumours displayed a conventional CD4+CD25+ Treg phenotype. Importantly, FOXP3+ TIL were positively correlated with CD8+ (cytotoxic) T cells (rs=0.76, P<0.0001), and were prognostically insignificant in tumours with low levels of CD8+ TIL. These observations were confirmed in an independent cohort.
Conclusion:
In contrast with current dogma, we show for the first time that FOXP3+ TIL are associated with robust anti-tumour immunity and favourable prognosis in ER– breast cancer.
Similar content being viewed by others
Tumour-infiltrating lymphocytes (TIL) are associated with patient survival in a wide variety of tumour types (Pages et al, 2010). In the case of breast cancer, several studies have demonstrated that gene expression patterns indicative of high TIL content are associated with improved clinical outcome, particularly among tumours with aggressive clinical features such as high histological grade or oestrogen receptor-α (ERα)-negative status (Teschendorff et al, 2007; Desmedt et al, 2008; Schmidt et al, 2008; Calabro et al, 2009; Rody et al, 2009). Several groups have extended these observations by using immunohistochemistry (IHC) to demonstrate that high levels of CD8+ TIL (cytotoxic T cells (CTLs)) are associated with good outcome in breast cancer (Mahmoud et al, 2011a; Liu et al, 2012). The opposite, however, appears to be true of FOXP3+ TIL.
FOXP3 is a forkhead family transcription factor that is essential for the development and function of regulatory T cells. Tregs are operationally identified by expression of CD4, CD25, and FOXP3, with FOXP3 being the most commonly used single marker (Campbell and Ziegler, 2007; Sakaguchi et al, 2008). Under normal conditions, Tregs are essential suppressors of inappropriate immune responses and thus maintain immunological tolerance to host tissues (Sakaguchi et al, 2008). Their suppression of anti-tumour immunity, however, is considered to be deleterious. Indeed, the presence of FOXP3+ TIL has been associated with poor clinical outcome in a wide variety of cancer types, fuelling speculation that depletion of Tregs in cancer patients could have beneficial therapeutic effects (deLeeuw et al, 2012). Nevertheless, a growing number of studies demonstrate that FOXP3+ TIL can also be associated with a favourable prognosis (reviewed in deLeeuw et al, 2012). Although this discordance is not currently understood, it is possible that the prognostic impact of FOXP3+ TIL depends on the molecular characteristics of a given tumour type.
To date, several studies of breast cancer have shown a consistent association between FOXP3+ TIL and poor clinical outcome (Bates et al, 2006; Aruga et al, 2009; Gobert et al, 2009; Liu et al, 2011; Yan et al, 2011; Mahmoud et al, 2011b). Although none of these studies focused on specific histological or molecular subtypes of breast cancer, FOXP3+ TIL showed a consistent association with ER-negative (ER–) tumours, which are biologically and clinically distinct from ER+ lesions (Foulkes et al, 2010). Because TIL have been shown to associate with good outcome primarily in ER– tumours (Teschendorff et al, 2007; Desmedt et al, 2008; Calabro et al, 2009; Rody et al, 2009), and because FOXP3+ TIL are inherently associated with ER negativity (Bates et al, 2006; Bohling and Allison, 2008; Ghebeh et al, 2008; Aruga et al, 2009; Liu et al, 2011; Mahmoud et al, 2011b; Yan et al, 2011), we chose to investigate the clinical impact of FOXP3+ TIL in ER– breast cancer. Importantly, we found that high numbers of FOXP3+ TIL are associated with dense infiltrates of CD8+ TIL and favourable prognosis.
Materials and methods
Cohort and sample selection
The primary cohort in this study has been previously described (West et al, 2011a). Briefly, formalin-fixed paraffin-embedded (FFPE) surgical breast tumour specimens were provided by the Manitoba Breast Tumour Bank (MBTB), which operates with approval from the Research Ethics Board, Faculty of Medicine, University of Manitoba. Cases for this study were originally accrued between the years 1988 and 2000 and had a median follow-up time of 83 months. Clinical characteristics of the study cohort are summarised in Supplementary Table S1. An initial cohort of 255 ER– cases was selected on the basis of (a) ER– status defined by ligand binding analysis of <10 fmol mg−1 protein, (b) invasive ductal carcinoma components occupying >20% of the tumour section, and (c) no neoadjuvant therapy. Recurrence-free survival (RFS) was defined as the time from surgery to the first instance of disease recurrence or death due to breast cancer, and disease-specific survival (DSS) was defined as the time to death due to breast cancer.
Tissue microarray construction and TIL assessment
Tissue microarray (TMA) construction and IHC was performed as described previously (Milne et al, 2009; West et al, 2011b) and in Supplementary Methods. Scoring for each IHC marker was undertaken by an experienced breast histopathologist (PHW) who was blinded to results of other markers or case identity. Prior use and exhaustion of some tissue cores reduced the interpretable cohort size to 175. A microscope eyepiece grid was used to standardise the assessed area of each tissue section (0.56 mm2 under × 20 magnification). Among duplicate tissue cores, those with an epithelial-stromal ratio closest to 1 : 1 were selected for further analysis. Positively stained lymphocytes (nuclear for FOXP3; cytoplasmic and/or membranous for CD8) were counted within the grid area and are expressed herein as cell numbers per mm2 of tissue.
Multicolour IHC
A second cohort comprising 14 cases of triple negative breast cancer collected between 2004 and 2007 was obtained from the BC Cancer Agency Tumour Tissue Repository, which operates with approval from the University of British Columbia and BC Cancer Agency Research Ethics Board. Multicolour IHC was performed as described in Supplementary Methods. Tumour-infiltrating lymphocytes were scored manually by two independent observers (NRW and SEK), with an interobserver Spearman’s correlation of rho>0.7.
Assessment of microarray data
Previously published (Prat et al, 2010) gene expression data from a third breast tumour cohort were downloaded from the University of North Carolina (UNC) microarray database. Only data corresponding to ER– cases were analysed. Data for selected genes were median normalised and converted to log2 ratios before analysis. Gene expression scores for differentiated CTLs, activated dendritic cells, and inflammatory cytokines were respectively calculated as the average expression values of CD247, CD8A, GZMB, IFNG; CD86, CD83, HLA-DRA; and IL18, IL1B, IL6, TNF. Similarly, scores for ligands of CCR5, CXCR3, and CCR4 were respectively calculated as the average expression of CCL3, CCL4, CCL5, CCL8; CXCL4, CXCL9, CXCL10, CXCL11; and CCL17, CCL22.
Statistical analyses
All univariate analyses were performed using Prism 5.0 (GraphPad, La Jolla, CA, USA). Multivariate Cox proportional hazards regression models were constructed using Statistics 14 (SPSS, Chicago, IL, USA). The TIL distributions showed significant skewness based on the D’Agostino and Pearson omnibus normality test (P<0.0001), and non-parametric tests were thus used where appropriate. Associations between TIL and clinical/pathological parameters were evaluated using Fisher’s exact tests or χ2 tests. The TIL distributions were compared using the Mann–Whitney U-test and Spearman’s correlation test. Univariate survival analyses were conducted using conventional Kaplan–Meier methods and log-rank tests. All tests were two-sided with significance established at P<0.05
X-Tile software (Camp et al, 2004) was used to identify an appropriate cutoff value for the definition of high vs low levels of FOXP3+ TIL. X-Tile creates equally sized training and validation cohorts with equivalent baseline hazard rates from a single data set. For a given variable, an optimal cutpoint that best discriminates good and poor clinical outcome is identified for the training cohort and subsequently tested in the validation cohort.
Results
High levels of FOXP3+ TIL are associated with favourable clinical outcome in ER– breast cancer
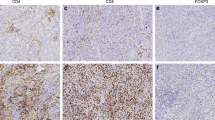
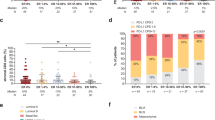
FOXP3+ TIL were detectable in 143 of 175 (82%) assessable tumours. The median number of FOXP3+ TIL was 9 per mm2, with respective lower and upper quartiles of 3 per mm2 and 27 per mm2. FOXP3+ lymphocytes were present throughout tumours (Figure 1A), with a tendency for stromal localisation (Figure 1B). When patients were categorised based on the presence or absence of recurrent disease at the time of final follow-up, FOXP3+ TIL were significantly more abundant in the disease-free subgroup (P=0.0033, Figure 1C).
Association of FOXP3+ TIL with patient survival. (A) Representative immunohistochemical staining of FOXP3 (brown nuclei), with examples of intraepithelial (arrowhead) and stromal (arrows) FOXP3+ TIL. Original magnification, × 200. (B) Box plots representing FOXP3+ TIL levels among tumour epithelia and stroma. (C) Distribution of FOXP3+ TIL in cases with (n=93) or without (n=82) disease recurrence at final follow-up. (D) Association of FOXP3+ TIL with RFS in the training, validation, and combined cohorts (FOXP3 high, n=92; FOXP3 low, n=83).
To establish a FOXP3 cutoff value for survival analysis, we used X-Tile software (Camp et al, 2004; as described in Materials and methods) and identified an optimal cutpoint of 18 FOXP3+ TIL per mm2. Cases with ⩾18 FOXP3+ TIL per mm2 had significantly prolonged RFS in both the training (HR=0.438, 95% CI 0.244–0.789; P=0.0059; Figure 1D) and validation cohorts (HR=0.490, 95% CI 0.275–0.873; P=0.0155). In the combined total cohort, the 10-year RFS rates were 59% and 24% for FOXP3-high and FOXP3-low patients, respectively (HR=0.461, 95% CI 0.306–0.696; P=0.0002). The cutoff value of 18 per mm2 was used to define FOXP3 status in all subsequent analyses.
To confirm the association between FOXP3+ TIL and patient outcome, we assessed several cutoff values that centred on 18 per mm2 (Supplementary Table S2). FOXP3+ TIL were significantly associated with RFS at cutoff values ranging from 5 per mm2 to 30 per mm2. The pattern of stromal vs intraepithelial infiltration did not significantly influence the clinical relevance of FOXP3+ TIL (data not shown).
The clinical significance of FOXP3+ TIL is restricted to high-grade tumours
When compared with standard clinical parameters, FOXP3+ TIL showed significant associations with younger patient age (P=0.0176), high tumour grade (P=0.0003), and treatment with adjuvant chemotherapy (P=0.0064; Supplementary Table S1). Given a recent report that CD8+ TIL are clinically relevant only in high-grade breast tumours (Baker et al, 2011), we assessed the importance of grade on the clinical relevance of FOXP3+ TIL (Supplementary Figure S2). Strikingly, FOXP3+ TIL failed to associate significantly with RFS in low/intermediate grade tumours (HR=0.703, 95% CI 0.371–1.333; P=0.2812), but did so strongly in the high-grade subset (HR=0.355, 95% CI 0.189–0.667; P=0.0013).
FOXP3+ TIL show strong clinical significance in triple negative and basal-like breast tumours
To determine whether FOXP3+ TIL levels vary within different molecular subtypes, we categorised cases based on Her2 overexpression, triple negative status, or basal-like histology (Supplementary Figure S3). The levels of FOXP3+ TIL were similar among these subtypes (P=0.7235, ANOVA). Intriguingly, FOXP3+ TIL were clinically irrelevant in the Her2+ subset (RFS, P=0.4928; DSS, P=0.3131), but were strongly associated with favourable RFS (HR=0.371, 95% CI 0.213–0.644; P=0.0004) and DSS (HR=0.416, 95% CI 0.231–0.750; P=0.0036) in triple negative tumours. This was particularly striking among basal-like tumours (RFS, HR=0.280, 95% CI 0.148–0.532; P=0.0001; and DSS, HR=0.281, 95% CI 0.142–0.556; P=0.0003; Supplementary Figure S3).
When the relative associations of standard clinical parameters with RFS were assessed in multivariate analysis, only lymph-node metastasis, tumour size, and treatment with chemotherapy emerged as significant independent prognostic parameters (Table 1). When corrected for these variables in the total cohort, FOXP3 status did not reach significance as a correlate of RFS (P=0.082). However, FOXP3 status showed independent statistical significance in the triple negative (P=0.041) and basal-like tumour subsets (P=0.010; Table 1).
The majority of FOXP3+ TIL in triple negative breast tumours co-express CD4 and CD25
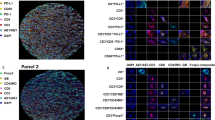
Our observation that FOXP3+ TIL are associated with good prognosis is inconsistent with the conventional assumption that they suppress anti-tumour immunity. Notably, effector T cells can transiently express low levels of FOXP3 following activation (Allan et al, 2007). To determine if this could explain our results, we performed multicolour IHC for detection of FOXP3, CD4, CD8, and CD25. Among a panel of 14 triple negative breast tumours, we found that, on average, 89% of FOXP3+ TIL expressed a CD4+CD8– phenotype (Figures 2A and C). Moreover, an average of 77% (range 60–100%) of FOXP3+ TIL co-expressed CD4 and CD25 (the classic Treg phenotype; Figures 2B and D). Therefore, the vast majority of FOXP3+ TIL in triple negative breast tumours are CD8–CD4+CD25+, supporting their classification as conventional Tregs.
Expression of CD4, CD25, and CD8 among FOXP3+ TIL in triple negative breast tumours. (A) Representative immunohistochemical (pseudocoloured) staining for FOXP3 (red), CD4 (green), and CD8 (blue). Examples of FOXP3+CD4+ (arrow), FOXP3–CD4+ (arrowhead), and FOXP3–CD8+ (asterisk) TIL are indicated. (B) Representative staining for FOXP3 (red), CD4 (green), and CD25 (blue). Examples of FOXP3+CD4+CD25+ (arrow) and FOXP3–CD4+CD25– (arrowhead) TIL are indicated. (C) Quantification of CD4 and CD8 expression and (D) CD4 and CD25 expression among FOXP3+ TIL for each tumour. Lines represent medians. *P<0.0001 vs all other groups, Mann–Whitney U-test.
FOXP3+ TIL are associated with tumour-infiltrating CTLs
Because CD8+ (cytotoxic) T cells are associated with a favourable prognosis in breast cancer (Baker et al, 2011; Ladoire et al, 2011; Liu et al, 2011, 2012; Mahmoud et al, 2011a), we hypothesised that high numbers of FOXP3+ lymphocytes may correlate with good outcome via an association with increased numbers of CD8+ TIL. Indeed, in the MBTB cohort we observed a strong correlation between FOXP3+ TIL and total CD8+ infiltrates (Spearman’s r=0.7606, 95% CI 0.6796–0.8233; P<0.0001; Figure 3A). This relationship was consistent regardless of whether CD8+ TIL were present in the tumour epithelium or stroma (Figure 3A), and was clearly evident within the basal-like subset (Spearman’s r=0.7995, 95% CI 0.6894–0.8735; P<0.0001). Notably, all FOXP3-high cases had detectable CD8+ TIL (Fisher’s exact test, P<0.0001).
Relationships between FOXP3+ TIL, cytotoxic T cells, and patient outcome. (A) Spearman’s correlation of FOXP3+ TIL vs total (left panel), intraepithelial (middle panel), and stromal (right panel) CD8+ TIL. The solid line represents the line of best fit, with 95% confidence intervals indicated as dashed lines. (B) RFS based on high (⩾upper quartile, n=37) or low (n=105) levels of intraepithelial CD8+ TIL (left panel), with box plots indicating FOXP3+ TIL levels in each group. (C, left panel) RFS among patients with high (⩾1, n=51) and low (<1, n=59) ratios of intraepithelial CD8+ to FOXP3+ TIL. Cases with no detectable FOXP3+ TIL were omitted. (C, right panel) Box plots representing FOXP3+ and intraepithelial CD8+ TIL levels in the indicated patient groups. (D) Association of FOXP3 (high n=30; low n=70) with RFS among patients with low levels of intraepithelial CD8+ TIL.
We have previously shown that intraepithelial (ie) CD8+ TIL are associated with good outcome in breast cancer (West et al, 2011a). In our current study, high levels (⩾36 per mm2, upper quartile) of ieCD8+ TIL were similarly associated with favourable RFS (HR=0.581, 95% CI 0.358–0.944; P=0.0283; Figure 3B). As we speculated, FOXP3+ TIL were significantly more abundant (>3-fold on average) in the ieCD8-high group compared with the ieCD8-low group (P<0.0001; Figure 3B).
In addition to high levels of CD8+ TIL, a high CD8+-to-FOXP3+ TIL ratio has been reported to associate with favourable prognosis in multiple tumour types (deLeeuw et al, 2012), including breast cancer (Ladoire et al, 2011; Liu et al, 2011). Among cases in our data set with detectable FOXP3+ TIL, we similarly observed that those with an ieCD8/FOXP3 ratio of ⩾1 had significantly better RFS than those with ratios <1 (P=0.0384; Figure 3C). However, while both groups had similar levels of FOXP3+ TIL, ieCD8+ TIL were significantly more abundant in the group with a high ieCD8/FOXP3 ratio (P<0.0001; Figure 3C). This suggests that the number of ieCD8+ TIL is a stronger prognostic factor than the ieCD8/FOXP3 ratio. Indeed, within the ieCD8/FOXP3-high group, the RFS of cases with low levels of ieCD8+ TIL (<36 per mm2) was indistinguishable from that of the ieCD8/FOXP3-low group (P=0.9871, data not shown).
If the relationship between FOXP3+ TIL and good clinical outcome is independent of CD8+ TIL, then one would expect FOXP3+ TIL to be prognostic in the absence of cytotoxic immune responses. However, high levels of FOXP3+ TIL were not significantly associated with RFS in tumours that were ieCD8 low (HR=0.728, 95% CI 0.426–1.246; P=0.2469; Figure 3D). Collectively, these data suggest that the association between FOXP3+ TIL and good prognosis in ER– breast cancer depends on the presence of CTLs.
Tregs are associated with good outcome and anti-tumour immunity in an independent data set
To test our observations in an independent cohort, we assessed gene expression data from a previously published study (Prat et al, 2010, UNC cohort). Because FOXP3 can be strongly expressed in epithelial cells (Merlo et al, 2009), we chose not to use this as an indicator of general Treg content. Rather, we used CD25, a gene that is expressed largely by lymphocytes and to a particularly strong extent by Tregs. Indeed, we found that an average of 80% of CD25+ lymphocytes in triple negative tumours were CD4+FOXP3+ (Supplementary Figure S4).
Among 78 ER– breast tumours, high (>median) CD25 expression was strongly associated with prolonged RFS (HR=0.279, 95% CI 0.135–0.550; P=0.0004; Figure 4A). High CD8A expression was also associated with good outcome (P=0.0019) as well as high CD25 expression (P=0.0002; Figure 4B). In the absence of high CD8A expression, however, CD25 had no prognostic significance (Figure 4C). Intriguingly, high CD25 expression was strongly associated with gene expression patterns indicative of differentiated CTLs (P<0.0001), activated dendritic cells (P<0.0001), and a pro-inflammatory cytokine milieu (P<0.0001; Figure 4D). CD25-high cases also displayed strong expression of ligands for the chemokine receptors CCR5 (P<0.0001) and CXCR3 (P=0.0012; Figure 4D), which are crucial for peripheral tissue infiltration by CTLs, Th1 cells, and Tregs (Mantovani et al, 2006; Ding et al, 2012; Oldham et al, 2012; Redjimi et al, 2012). In contrast, CCL17 and CCL22, which mediate the selective recruitment of Tregs (Curiel et al, 2004; Hirahara et al, 2006; Gobert et al, 2009), were not highly expressed in CD25-high tumours (P=0.2540; Figure 4D). Together with our IHC-based results, these data suggest that strong infiltration of ER– breast tumours by Tregs is indicative of robust anti-tumour immunity.
Association of Tregs with prognosis and immune parameters in an independent data set. (A) Five-year RFS of patients with high (n=38) and low (n=40) levels of CD25 expression. (B) Five-year RFS of patients with high (n=47) and low (n=47) levels of CD8A expression, with corresponding box plots indicating CD25 expression. (C) Association of CD25 (high n=11; low n=30) with RFS among patients with low CD8A expression. (D) Gene expression modules indicative of differentiated cytotoxic T cells, activated dendritic cells, inflammatory cytokines, and chemokines relative to CD25 status. In all panels, asterisks represent Mann–Whitney U-test significance levels as follows: ****P<0.0001, ***P=0.001–0.01, **P=0.01–0.05.
Discussion
While CTLs are associated with favourable clinical outcome in various tumour settings, the relationship between FOXP3+ TIL (putative Tregs) and prognosis is less clear. In some cancer types, such as hepatocellular carcinoma, FOXP3+ TIL are consistently associated with poor outcome, while in colorectal cancer the opposite is true (deLeeuw et al, 2012). In the case of breast cancer, six of seven studies report a relationship between FOXP3+ TIL and poor outcome (Bates et al, 2006; Aruga et al, 2009; Gobert et al, 2009; de Kruijf et al, 2010; Liu et al, 2011; Yan et al, 2011; Mahmoud et al, 2011b). Our observation that FOXP3+ TIL are associated with good outcome in ER– breast cancer thus seemingly contradicts the current balance of literature. However, ours is the first study to address the relationship between FOXP3+ TIL and outcome specifically in ER– breast cancer.
The ER– breast tumours are biologically and clinically distinct from their ER+ counterparts (Foulkes et al, 2010). Tumour-infiltrating lymphocytes are notably associated with good outcome among ER– tumours, but not typically among ER+ lesions (Teschendorff et al, 2007; Desmedt et al, 2008; Schmidt et al, 2008; Calabro et al, 2009; Rody et al, 2009). Furthermore, ER– tumours contain higher levels of TIL than ER+ tumours (Bates et al, 2006; Ghebeh et al, 2008; Foulkes et al, 2010; Baker et al, 2011; Liu et al, 2011, 2012; Mahmoud et al, 2011a, 2011b). Because prior studies of FOXP3+ TIL in breast cancer involved mixed cohorts that were largely comprised of ER+ cases, the relationship between FOXP3+ TIL and good prognosis in ER– disease may have been obscured by a negative prognostic relationship among ER+ tumours. For example, Bates et al (2006) and Mahmoud et al (2011b) reported that FOXP3+ TIL were associated with poor prognosis in ER+ breast cancer, but apparently not among ER– cases. Importantly, the FOXP3 cutoff values used in these prior studies may not have reflected clinically relevant FOXP3+ TIL levels within ER– tumours. For example, if the cutoff values used in the Bates or Mahmoud studies (respectively, 5 per mm2 (35th percentile) and 0 per mm2 (18th percentile)) are applied to our data, we obtain at best a weak discrimination of patient survival (Supplementary Table S2). By examining a strictly ER– cohort, our observations may be explained by a FOXP3 cutoff value that is more relevant to ER– tumours.
Beyond considerations of tumour subtype, we also examined the possibility that a significant proportion of FOXP3+ TIL in our cohort might be effector T cells undergoing transient activation-induced FOXP3 expression. This was untrue, however, as over three quarters of FOXP3+ TIL displayed a classic CD4+CD25+ phenotype in triple negative tumours (Figure 2). Rather, FOXP3+ TIL were strongly associated with CD8+ TIL, which are known correlates of good outcome in breast cancer (Baker et al, 2011; Mahmoud et al, 2011a; West et al, 2011a). Importantly, the association of FOXP3+ TIL with good outcome was dependent on the presence of large numbers of CD8+ TIL (Figure 3D). These results were confirmed in the UNC data set (using CD25 as an alternative Treg marker). Therefore, in the context of ER– breast cancer, FOXP3+ TIL appear to serve as markers of robust anti-tumour immunity.
Our findings could be explained by the fact that CD8+ T cells and Tregs infiltrate tumours using similar mechanisms (Fisher et al, 2006; Ley et al, 2007). For T-cell extravasation to occur, T cells must initiate vascular rolling by interacting with endothelial selectins via CD44, CD62L, or PSGL, which are expressed by both Tregs and activated CD8+ T cells (Ohmichi et al, 2011). Chemokines on the vascular lumen then activate lymphocyte adhesion molecules to promote extravasation (Ley et al, 2007). Importantly, the majority of chemokine receptors that mediate CD8+ T-cell extravasation (e.g., CCR5, CXCR3, and CXCR6) are also expressed by FOXP3+ T cells (Ding et al, 2012; Oldham et al, 2012; Redjimi et al, 2012). As such, it is logical that FOXP3+ and CD8+ TIL co-infiltrate breast tumours. Indeed, CD25-high tumours in the UNC data set expressed high levels of ligands for CCR5 and CXCR3 (Figure 4D), but not CCL22, which selectively recruits Tregs via CCR4 (Curiel et al, 2004; Gobert et al, 2009). Notably, some breast tumours appear to contain high endothelial venules (HEVs), blood vessels that specialise in lymphocyte recruitment and are normally found in lymphoid organs (Martinet et al, 2011). In human breast tumours, HEVs are strongly correlated with a wide variety of TIL types, suggesting that HEVs could serve as a shared route of infiltration for CD8+ and FOXP3+ TIL.
It is intriguing that CD8+ TIL are associated with good outcome in ER– breast cancer despite a large number of co-infiltrating Tregs, which are conventionally expected to suppress immunity. In general, Tregs require close contact with target cells to exert suppression (Shevach, 2009). However, in the triple negative cohort assessed in our study, fewer than 20% of CD4+FOXP3+ lymphocytes were in direct contact with CD8+ TIL (data not shown), suggesting that Tregs in ER– breast tumours may not exert significant suppression on CTLs. Moreover, gene expression data from the UNC cohort demonstrated that markers of dendritic cell activation, CD8+ T-cell cytotoxicity, and inflammatory cytokine production were enriched in CD25-high tumours, suggesting that multiple important aspects of anti-tumour immunity can be active in ER– breast tumours despite the presence of Tregs.
While this is the first report of FOXP3+ TIL being associated with good outcome in ER– breast cancer, this relationship has been observed in other tumour types, notably colorectal and ovarian cancer (Milne et al, 2009; Salama et al, 2009; Frey et al, 2010; Zlobec et al, 2010; Yoon et al, 2012). In both these settings, FOXP3+ TIL are strongly correlated with high levels of CD8+ TIL, in agreement with our own results (Milne et al, 2009; Salama et al, 2009; Yoon et al, 2012). In the context of colon cancer, Tregs may be capable of exerting pro-inflammatory Th17-like effects (Blatner et al, 2010). In addition, Tregs could conceivably suppress or eliminate innate leukocytes (such as macrophages) that are thought to promote tumour progression through the production of mitogens and angiogenic factors (DeNardo et al, 2008). This highlights the possibility that FOXP3+ TIL may have direct host-protective effects in ER– breast tumours. Alternatively, Tregs may have normal suppressive capacity, but at levels insufficient to override the anti-tumour activity of CD8+ TIL. In this case, eliminating Tregs from ER– breast tumours could enhance anti-tumour immunity beyond the already beneficial natural response.
To determine if Tregs in ER– breast tumours are beneficial, deleterious, or inconsequential, their phenotypic and functional qualities must be assessed more comprehensively. Given that the prognostic impact of Tregs appears to differ based on ER status, histological grade, and molecular subtype, comparing the biology of Tregs in distinct tumour phenotypes may provide valuable insights into both Treg biology and breast cancer immunology. Until we have a better understanding of the functional properties of FOXP3+ TIL in breast cancer, it seems premature to proceed with strategies aimed at depleting or inhibiting these cells in patients.
Change history
15 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK (2007) Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19 (4): 345–354
Aruga T, Suzuki E, Saji S, Horiguchi S, Horiguchi K, Sekine S, Kitagawa D, Funata N, Toi M, Sugihara K, Kuroi K (2009) A low number of tumour-infiltrating FOXP3-positive cells during primary systemic chemotherapy correlates with favourable anti-tumour response in patients with breast cancer. Oncol Rep 22 (2): 273–278
Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD (2011) Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology 58 (7): 1107–1116
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24 (34): 5373–5380
Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ, Khazaie K (2010) In colourectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA 107 (14): 6430–6435
Bohling SD, Allison KH (2008) Immunosuppressive regulatory T cells are associated with aggressive breast cancer phenotypes: a potential therapeutic target. Mod Pathol 21 (12): 1527–1532
Calabro A, Beissbarth T, Kuner R, Stojanov M, Benner A, Asslaber M, Ploner F, Zatloukal K, Samonigg H, Poustka A, Sultmann H (2009) Effects of infiltrating lymphocytes and oestrogen receptor on gene expression and prognosis in breast cancer. Breast Cancer Res Treat 116 (1): 69–77
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10 (21): 7252–7259
Campbell DJ, Ziegler SF (2007) FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol 7 (4): 305–310
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10 (9): 942–949
de Kruijf EM, van Nes JG, Sajet A, Tummers QR, Putter H, Osanto S, Speetjens FM, Smit VT, Liefers GJ, van de Velde CJ, Kuppen PJ (2010) The predictive value of HLA class I tumour cell expression and presence of intratumoural Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res 16 (4): 1272–1280
deLeeuw RJ, Kost SE, Kakal JA, Nelson BH (2012) The prognostic value of FoxP3+ tumour-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 18 (11): 3022–3029
DeNardo DG, Johansson M, Coussens LM (2008) Immune cells as mediators of solid tumour metastasis. Cancer Metastasis Rev 27 (1): 11–18
Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, Delorenzi M, Piccart M, Sotiriou C (2008) Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 14 (16): 5158–5165
Ding Y, Xu J, Bromberg JS (2012) Regulatory T cell migration during an immune response. Trends Immunol 33 (4): 174–180
Fisher DT, Chen Q, Appenheimer MM, Skitzki J, Wang WC, Odunsi K, Evans SS (2006) Hurdles to lymphocyte trafficking in the tumour microenvironment: implications for effective immunotherapy. Immunol Invest 35 (3-4): 251–277
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363 (20): 1938–1948
Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L (2010) High frequency of tumour-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colourectal cancer patients. Int J Cancer 126 (11): 2635–2643
Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S (2008) FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumour tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer 8: 57
Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Menetrier-Caux C (2009) Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumours and lead to an adverse clinical outcome. Cancer Res 69 (5): 2000–2009
Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS (2006) The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol 177 (7): 4488–4494
Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, Coudert B, Martin F, Bizollon MH, Vanoli A, Coutant C, Fumoleau P, Bonnetain F, Ghiringhelli F (2011) In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol 224 (3): 389–400
Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7 (9): 678–689
Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, Yin D, Gu F, Yao Z, Fu L (2011) CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 130 (2): 645–655
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO (2012) CD8+ lymphocyte infiltration is an independent favourable prognostic indicator in basal-like breast cancer. Breast Cancer Res 14 (2): R48
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR (2011a) Tumour-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29 (15): 1949–1955
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR (2011b) An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat 127 (1): 99–108
Mantovani A, Bonecchi R, Locati M (2006) Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol 6 (12): 907–918
Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP (2011) Human solid tumours contain high endothelial venules: association with T- and B-lymphocyte infiltration and favourable prognosis in breast cancer. Cancer Res 71 (17): 5678–5687
Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, Tagliabue E, Balsari A (2009) FOXP3 expression and overall survival in breast cancer. J Clin Oncol 27 (11): 1746–1752
Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH (2009) Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 4 (7): e6412
Ohmichi Y, Hirakawa J, Imai Y, Fukuda M, Kawashima H (2011) Essential role of peripheral node addressin in lymphocyte homing to nasal-associated lymphoid tissues and allergic immune responses. J Exp Med 208 (5): 1015–1025
Oldham KA, Parsonage G, Bhatt RI, Wallace DM, Deshmukh N, Chaudhri S, Adams DH, Lee SP (2012) T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur Urol 61 (2): 385–394
Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH (2010) Immune infiltration in human tumours: a prognostic factor that should not be ignored. Oncogene 29 (8): 1093–1102
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12 (5): R68
Redjimi N, Raffin C, Raimbaud I, Pignon P, Matsuzaki J, Odunsi K, Valmori D, Ayyoub M (2012) CXCR3+ T regulatory cells selectively accumulate in human ovarian carcinomas to limit type I immunity. Cancer Res 72 (17): 4351–4360
Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, Engels K, Karn T, Kaufmann M (2009) T-cell metagene predicts a favourable prognosis in oestrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res 11 (2): R15
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133 (5): 775–787
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B (2009) Tumour-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colourectal cancer. J Clin Oncol 27 (2): 186–192
Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, Lehr HA, Hengstler JG, Kolbl H, Gehrmann M (2008) The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 68 (13): 5405–5413
Shevach EM (2009) Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30 (5): 636–645
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C (2007) An immune response gene expression module identifies a good prognosis subtype in oestrogen receptor negative breast cancer. Genome Biol 8 (8): R157
West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH (2011a) Tumour-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in oestrogen receptor-negative breast cancer. Breast Cancer Res 13 (6): R126
West NR, Panet-Raymond V, Truong PT, Alexander C, Babinszky S, Milne K, Ross LA, Loken S, Watson PH (2011b) Intratumoural immune responses can distinguish new primary and true recurrence types of ipsilateral breast tumour recurrences (IBTR). Breast Cancer (Auckl) 5: 105–115
Yan M, Jene N, Byrne D, Millar EK, O'Toole SA, McNeil CM, Bates GJ, Harris AL, Banham AH, Sutherland RL, Fox SB (2011) Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res 13 (2): R47
Yoon HH, Orrock JM, Foster NR, Sargent DJ, Smyrk TC, Sinicrope FA (2012) Prognostic impact of FoxP3+ regulatory t-cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS ONE 7 (8): e42274
Zlobec I, Karamitopoulou E, Terracciano L, Piscuoglio S, Iezzi G, Muraro MG, Spagnoli G, Baker K, Tzankov A, Lugli A (2010) TIA-1 cytotoxic granule-associated RNA binding protein improves the prognostic performance of CD8 in mismatch repair-proficient colourectal cancer. PLoS ONE 5 (12): e14282
Acknowledgements
We thank Sindy Babinszky (BC Cancer Agency Tumour Tissue Repository) for technical assistance and members of the Deeley Research Centre for helpful discussion. This study was supported by the BC Cancer Agency Tumour Tissue Repository and the Manitoba Breast Tumour Bank, both members of the Canadian Tumour Repository Network. Funding was from the Canadian Institutes of Health Research (CIHR grant MOP-64349), the Canadian Breast Cancer Foundation, and the BC Cancer Foundation. NRW and SEK were respectively supported by training grants from the US DOD breast cancer research program (W81XWH-08-1-0781) and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
West, N., Kost, S., Martin, S. et al. Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer 108, 155–162 (2013). https://doi.org/10.1038/bjc.2012.524
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.524
Keywords
This article is cited by
-
The prognostic value of tumour-infiltrating lymphocytes, programmed cell death protein-1 and programmed cell death ligand-1 in Stage I–III triple-negative breast cancer
British Journal of Cancer (2023)
-
Tumour-infiltrating lymphocytes: from prognosis to treatment selection
British Journal of Cancer (2023)
-
The prognostic values of FOXP3+ tumor-infiltrating T cells in breast cancer: a systematic review and meta-analysis
Clinical and Translational Oncology (2023)
-
Comprehensive Analysis of Prognostic and immune infiltrates for FOXPs Transcription Factors in Human Breast Cancer
Scientific Reports (2022)
-
Identification of a 5-gene-risk score model for predicting luminal A-invasive lobular breast cancer survival
Genetica (2022)