Abstract
Background:
Over 8000 new pancreatic cancers are diagnosed annually in the UK; most at an advanced stage, with only 3% 5-year survival. We aimed to identify and quantify the risk of pancreatic cancer for features in primary care.
Methods:
A case–control study using electronic primary care records identified and quantified the features of pancreatic cancer. Cases, aged ⩾40 in the General Practice Research Database, UK, with primary pancreatic cancer were matched with controls on age, sex and practice. Putative features of pancreatic cancer were identified in the year before diagnosis. Odds ratios (OR) were calculated for features of cancer using conditional logistic regression. Positive predictive values (PPV) were calculated for consulting patients.
Results:
In all, 3635 cases and 16 459 controls were studied. Nine features were associated with pancreatic cancer (all P<0.001 except for back pain, P=0.004); jaundice, OR 1000 (95% confidence interval (CI) 4 302 500); abdominal pain, 5 (4.4, 5.6); nausea/vomiting, 4.5 (3.5, 5.7); back pain, 1.4 (1.1, 1.7); constipation, 2.2 (1.7, 2.8); diarrhoea, 1.9 (1.5, 2.5); weight loss, 15 (11, 22); malaise, 2.4 (1.6, 3.5); new-onset diabetes 2.1 (1.7, 2.5). Positive predictive values for patients aged ⩾60 were <1%, apart from jaundice at 22% (95% CI 14, 52), though several pairs of symptoms had PPVs >1%.
Conclusion:
Most previously reported symptoms of pancreatic cancer were also relevant in primary care. Although predictive values were small – apart from jaundice – they provide a basis for selection of patients for investigation, especially with multiple symptoms.
Similar content being viewed by others
Main
Pancreatic tumours account for 2.6% of all newly diagnosed cancers in the UK with ∼8000 new diagnoses and deaths each year, making it the fifth most common cause of cancer death in the UK (Cancer Research UK, 2008). Worldwide there are an estimated 279 000 new cases per year. It has a very poor prognosis, with few patients eligible for potentially curative surgery, leading to a 5-year survival of around 3% (Rachet et al, 2009). The incidence increases with age, with over 75% of new cases occurring in those aged 65 and over. Most tumours originate from the exocrine part of the pancreas. Endocrine tumours are rare but generally have a much more favourable prognosis. Despite the emergence of promising biomarkers for diagnosis there are no screening tests currently available, so diagnosis depends upon presentation with symptoms.
The symptoms of primary pancreatic cancer have only been described in secondary care studies. It occurs rarely in primary care: a full time general practitioner (GP) would see on average only one case every 5 years (NICE, 2001). Studies have mostly been retrospective surgical case series, with the potential for recall bias; perforce, they study patients late in the natural course of their disease. Abdominal pain is reported in up to 80% of patients (Takhar et al, 2004). Obstructive jaundice, which occurs when the biliary tract becomes blocked by the tumour, occurs in 16–85%: (Klamer and Max, 1982; Mannell et al, 1986; Manabe et al, 1988; Furukawa et al, 1996; Wilson et al, 2000; Elli et al, 2003) it is usually a feature of advanced disease. Weight loss is also reported in 3–82%, again generally denoting advanced disease (Klamer and Max, 1982; Mannell et al, 1986; Furukawa et al, 1996; Elli et al, 2003). Pancreatitis and thrombo-phlebitis have also been described (BakkevoldK et al, 1992; Picozzi, 2005) Diabetes may follow if pancreatic damage is considerable (Everhart and Wright, 1995). Other symptoms, such as loss of taste, pruritus, psychological disturbances or skin changes, have been reported for up to 2 years before onset of pain or jaundice (Picozzi, 2005).
UK guidance for the selection of patients for urgent investigation of suspected cancer has no specific section relating to pancreatic cancer: instead, recommendations for oesophageal, gastric and pancreatic cancer are merged, with criteria for upper gastrointestinal endoscopy predominant (NICE, 2005). Symptoms potentially representing one of these three cancer sites recommended for urgent investigation are obstructive jaundice, persistent vomiting, anaemia or an unexplained loss of weight. In a recent audit of 345 upper gastrointestinal urgent referrals based on these recommendations in London, 36 (11.4%) upper gastrointestinal cancers were identified, 13 being pancreatic (Patel et al, 2011). Most were advanced disease. The authors called for better selection of patients for investigation: this requires study of the symptoms of pancreatic cancer in primary care. We sought to identify features of pancreatic cancer, and to quantify their risk, in patients presenting to primary care.
Materials and methods
This was a case–control study using data from the General Practice Research Database (GPRD) in the UK. The GPRD maintains an anonymised copy of participating practices’ medical records: these contain full details of the patient, including all consultations, recorded symptoms, investigations and diagnoses. The data are subject to stringent checks on validation and quality, and they are regarded as high quality in terms of accuracy and completeness and validity of diagnoses (Herrett et al, 2010; Khan et al, 2010). We have previously used similar methods for several cancer diagnostic studies (Hamilton, 2009).
Identification of cases and controls
A list of 25 pancreatic tumour diagnostic codes (available from the authors) was collated from the GPRD master code library. This has ∼100 000 codes covering all events in primary care. Some codes specifically identified the histological subtype, but most were unspecified, such as ‘malignant neoplasm of pancreas’. Staff from the GPRD identified all patients aged 40 years and over with a pancreatic tumour diagnosed between 1st January, 2000 and 31st December, 2009 and with at least 1 year of data meeting their quality standards before the first diagnostic code. For each case, the GPRD identified up to five controls, matched to the case by year of birth, sex and practice and randomly selected using a computer-generated sequence. We chose five controls for maximum power within the GPRD’s constraints as to total database size. We excluded cases and controls if they had no consultations in the year before diagnosis in the case (the index date). Controls were excluded if they had ever had pancreatic cancer.
Selection of possible features of pancreatic cancer
We studied all symptoms, physical signs or abnormal investigations compiled from the pancreatic cancer literature, supplemented by discussion with two pancreatic cancer charities (full list available from authors). For simplicity, these are called ‘features’ from now on. Libraries of codes relating to these were collated. We also identified all codes for fractures, as a test for any recording bias between cases and controls (making the assumption that the fracture rate would be approximately equal). Occurrences of these features in the year before the index date were identified. Features were only retained for further study if they occurred in ⩾5% of cases or controls. Repeat attendances with the same symptom were also retained if the subsequent consultation also occurred in ⩾5% of cases or controls. We defined new-onset diabetes as a code for diabetes, or a random blood glucose above the local laboratory’s normal range, without similar codes more than 1 year before the index date. For laboratory tests, we considered patients without a test to be the same status as those with a normal result, making our binary variable abnormal result/ no abnormal result. We defined abnormal liver function as any liver enzyme above the normal range, and raised inflammatory markers as either abnormal erythrocyte sedimentation rate or C-reactive protein, as there were too few plasma viscosity results.
Analysis
Univariable analysis of differences between cases and controls was undertaken using conditional logistic regression to account for the matching. Variables independently associated with pancreatic cancer with a P-value <0.1 were entered into multivariable analyses. Multivariable regressions were performed in stages, initially collecting similar variables together, such as those reflecting abdominal pain. Significant variables then entered a second stage, grouping variables into abdominal symptoms, other symptoms, physical signs and investigations. For these two stages, a threshold P-value of <0.05 was used. The final model was derived from all variables surviving the earlier stage regressions, and used a threshold P-value of <0.01. All rejected variables were checked to see if they added anything to the final model. Lastly, nine clinically plausible interactions were tested.
Calculation of positive predictive values (PPVs)
It was possible to calculate PPVs for the risk of pancreatic cancer in patients consulting in primary care using Bayes’ theorem (Knottnerus, 2002). In this, the posterior odds of disease=the prior odds × the likelihood ratio. For the prior odds we used the age-specific national incidence rate of pancreatic cancer for 2008 (Cancer Research UK, 2008). As all 3635 cases analysed had consulted in primary care, but only 16 459 of 17 913 (92.1%) controls had consulted in the study period, we divided the posterior odds by 0.921 to give predictive values for the consulting population. Analyses were performed using Stata (version 11) (Stata Statistical Software, 2010).
Results
We were initially supplied with 3647 cases and 17 977 controls, as 1207 cases could not be matched to five controls. The application of exclusions is shown in Figure 1.
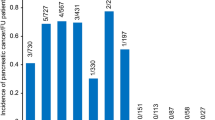
Of the 3635 cases included in the analyses, 98 (2.7%) had a specific code for an endocrine tumour, though 2342 (64.2%), had a generic label and some of these may have been endocrine. The demographic features of cases are shown in Table 1, and the univariable results for selected clinical features are shown in Table 2. Sixty five (1.8%) of cases and 297 (1.8%) of controls had a record of a fracture. In the year before diagnosis, cases presented to primary care twice as frequently as controls: cases median 18; interquartile range (IQR) 11, 27: controls median 9 (IQR) 4, 15.
Multivariable analysis results are shown in Table 3. One antagonistic interaction term is included: abdominal pain with jaundice. This can probably be explained clinically by an alternative diagnosis of gallstones, which also may have abdominal pain and jaundice as presenting features. External interaction terms (testing whether features were more or less strongly associated with cancer according to age, sex and smoking habits) were not significant.
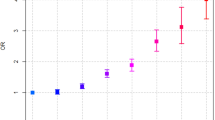
Figure 2 shows PPVs for pancreatic cancer for the symptoms shown to be independently associated in multivariable analysis – individually, when repeated and in combination with another symptom for patients aged ⩾60 years of either sex. This age was chosen to allow over 80% of the pancreatic cancer population to be shown in a single figure. For jaundice with constipation, diarrhoea, loss of weight or malaise, more than 40 cases but no controls had the combination; while strictly speaking undefined, the PPV must logically be very high and so it has been set as >10%. One subgroup analysis by age was undertaken. Patients aged ⩾70 (the older half of the cohort) had PPVs for most symptoms 1.5 to 4.5 times higher than those for patients under 70 years of age.
Positive predictive values (95% CIs) for pancreatic cancer in men and women aged over 60 for individual risk markers and for pairs of risk markers in combination. (1) The top figure in each cell is the positive predictive value when both features are present. The two smaller figures represent the 95% CIs for the positive predictive value. These have not been calculated when any cell in the 2 × 2 table was below 5 (invariably this was because too few controls had both features). (2) The yellow shading is for pairs of symptoms with a positive predictive value over 1%; the amber shading is when the positive predictive value is above 2.0%, and the red shading is for positive predictive values above 5.0%. (3) The jaundice/jaundice intersect is the positive predictive value for pancreatic cancer when a patient has attended at least twice with jaundice. The same is true for abdominal pain and back pain.
Discussion
Summary of main findings
This is the first study of the clinical features of pancreatic cancer in primary care. Most of the symptoms reported from secondary care studies were also strongly associated with pancreatic cancer in primary care. However, the risk of pancreatic cancer with these features – other than jaundice – was low, reflecting the rarity of pancreatic tumours and that many of the symptoms are common in benign conditions. The risk of an underlying pancreatic cancer with these symptoms was higher in older patients, and in patients with multiple symptoms.
Strengths and limitations of the study
This study is large, and uses primary care data. This is crucial: selection of patients for investigation is performed by clinicians in primary care, so primary care data must be used to illuminate the selection process. The GPRD is considered by many to be the 'gold standard' of longitudinal patient databases from primary care. It has been used in nearly 1000 research papers published in peer-reviewed journals and its validity has been well documented (Jick et al, 1991; Jick et al, 2003; Fombonne et al, 2004; Herrett et al, 2010; Khan et al, 2010). The patient population in the database is also broadly representative of the UK population. Additionally, laboratory results are transmitted directly to the database, allowing us to use the local normal range to identify abnormal results.
We could not check the accuracy of diagnosis in the cases by histology, or determine the staging. Linkage to cancer registries is now possible, though only for a part of the GPRD. Most cases had multiple records of a pancreatic neoplasm. It is unlikely that such a serious disease would be recorded incorrectly with any frequency.
The most significant limitation of the study is that we had to rely upon the accurate recording by GPs of both symptoms and diagnoses. Under-recording of symptoms or signs may have led to some features, which may be genuinely associated with pancreatic cancer, such as deep vein thrombosis or steatorrhoea, not being identified in this study. One particular concern is that symptoms may be recorded in an inaccessible part of the GPRD – the so-called ‘free-text’ area. This is possible, though a recent study of ovarian cancer identified relatively little hidden data in these fields (Tate et al, 2011). However, when calculating likelihood ratios and PPVs, under-recording is only important if the proportion of under-recording was differentially higher in either cases or controls. We have no evidence to suggest this is the case.
Comparison with existing literature
The prevalence of the most common symptoms of pancreatic cancer was lower than in previous secondary care studies (Krech and Walsh, 1991; Bakkevold et al, 1992; Gullo et al, 2001; Holly et al, 2004; Porta et al, 2005). This may well reflect different symptom experience early in the disease – especially in primary care. There are two possible alternative explanations. The first is under-recording of symptoms, as discussed earlier; the second is that most previous studies directly interviewed patients after diagnosis. Such methods may be subject to recall bias and usually uncover higher rates of symptom reporting than do studies using indirect methods such as ours. One UK questionnaire-based study, which compared symptom recording in pancreatic disease by GPs to symptom recording by health professionals in secondary care, found prevalence figures similar to this study (Virlos et al, 2005). Our finding of more frequent attendances mirrors reports in the National Cancer patient Experience Survey, where 41% of pancreatic cancer patients reported consulting their GP at least three times before diagnosis (Lyratzopoulos et al, 2012).
Implications for clinical practice and future research
Our results should help GPs decide which patients to refer for further investigation. A fifth of patients over 60 years with recorded jaundice will have pancreatic cancer: this figure would justify urgent referral. Current NICE guidance recommends urgent referral only for obstructive jaundice, but the results from this study would suggest that investigation for jaundice per se is warranted, unless there is a clear alternative cause (NICE, 2005). Even if no malignancy is uncovered by investigation, a common alternative explanation for jaundice is stones in the biliary system, and these are also worth identifying without undue delay. Similarly, loss of weight had moderate PPVs when accompanied by a second symptom from Table 3. Arguably, such patients could also be considered for investigation. One problem is that ultrasonography is excellent at visualising the biliary tree, but CT scanning is preferred for imaging the pancreas itself – thus, non-jaundiced patients would probably require the latter form of imaging. Although more expensive than ultrasonography, CT scanning may identify a cause for the symptoms should the pancreas appear normal.
Conclusion
Although current mortality from pancreatic cancer is very high in the UK, other European countries have better outcomes from the cancer (Abdel-Rahman et al, 2009). It is not known if these better outcomes arise from improved diagnostics or better treatment, or both, but it gives some hope that the current dismal prognosis can be improved somewhat. Our figures can guide GPs in selection of patients for urgent investigation. Even though no new major findings have emerged, this study provides primary care evidence for the forthcoming revision of the UK NICE referral guidance.
Change history
23 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abdel-Rahman M, Stockton D, Rachet B, Hakulinen T, Coleman MP (2009) What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable. Br J Cancer 101: S115–S124
Bakkevold KE, Arnesjo B, Kambestad B (1992) Carcinoma of the pancreas and papilla of Vater: presenting symptoms, signs, and diagnosis related to stage and tumour site. A prospective multicentre trial in 472 patients. Norwegian Pancreatic Cancer Trial. Scand J Gastroenterol 27: 317–325
Cancer Research UK (2008) Incidence Statistics (http://publications.cancerresearchuk.org/downloads/Product/cs_pdf_incidence_feb_2008.pdf)
Elli M, Piazza E, Franzone PC, Isabella L, Poliziani D, Taschieri AM (2003) Considerations on early diagnosis of carcinoma of the pancreas. Hepatogastroenterology 50: 2205–2207
Everhart J, Wright D (1995) Diabetes mellitus as a risk factor for pancreatic cancer. JAMA 273: 1605–1609
Fombonne E, Heavey L, Smeeth L, Rodrigues LC, Cook C, Smith PG, Meng L, Hall AJ (2004) Validation of the diagnosis of autism in general practitioner records. BMC Public Health 4 doi:10.1186/1471-2458-4-5
Furukawa H, Okada S, Saisho H (1996) Clinicopathologic features of small pancreatic adenocarcinoma; a collective study. Cancer 78: 986–990
Gullo L, Tomassetti P, Migliori M, Casadei R, Marrano D (2001) Do Early Symptoms of Pancreatic Cancer Exist that Can Allow an Earlier Diagnosis? Pancreas 22: 210–213
Holly EA, Chaliha I, Bracci PM, Gautam M (2004) Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol 2: 510–517
Hamilton W (2009) The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer S80–S86
Herrett E, Thomas S, Schoonen W, Smeeth L, Hall A (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 69: 4–14
Jick H, Jick S, Derby L (1991) Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. Br Med J 302: 766–768
Jick SS, Kaye JA, Vasilakis-Scaramozza C, Garcia Rodríguez LA, Ruigómez A, Meier CR, Schlienger RG, Black C, Jick H. (2003) Validity of the General Practice Research Database. Pharmacotherapy 23 (5): 686–689
Khan N, Harrison S, Rose P (2010) Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 60: e128–e136
Klamer T, Max M (1982) Pancreatic carcinoma. South Med J 75: 780–782
Knottnerus JA (2002) The evidence base of clinical diagnosis. BMJ Books: London
Krech RL, Walsh D (1991) Symptoms of pancreatic cancer. J Pain Symptom Manage 6: 360–367
Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA (2012) Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 13: 353–365
Manabe T, Miyashita T, Ohshio G (1988) Small carcinoma of the pancreas, clinical and pathological evaluation of 17 patients. Cancer 62: 135–141
Mannell A, Weiland L, van Heerden J, Ilstrup D (1986) Factors influencing survival after resection for ductal adenocarcinoma of the pancreas. Ann Surg 203: 403–407
NICE (2001) Guideline on the use of gemcitabine for the treatment of pancreatic cancer. Technology appraisal guidance no. 25 (〈http://www.nice.org.uk/newsroom/pressreleases/pressreleasearchive/pressreleases2001/2001_16_nice_issues_guidance_on_gemcitabine_for_pancreatic_cancer.jsp)
NICE (2005) Referral Guidelines for suspected cancer. NICE: London
Patel K, Perkins L, Mann S (2011) Are upper gastrointestinal cancer two week referrals an appropriate use of National Health Service resources? Clin Med 4: 412
Picozzi V (2005) In Pancreatic Cancer Daniel D Von Hoff, Douglas B Evans and Ralph H Hruban (eds) Jones and Bartlett Publishers: Sudbury, Massachusetts
Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, Ruiz L, Jariod M, Costafreda S, Coll S, Alguacil J, Corominas JM, Solà R, Salas A, Real FX. (2005) Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 7: 189–197
Rachet B, Maringe C, Nur U, Quaresma M, Shah A, Woods LM, Ellis L, Walters S, Forman D, Steward J, Coleman MP (2009) Population-based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncol 10: 351–369
Stata Statistical Software: Release 11, (2010). Stata Corporation: College Station, TX
Takhar AS, Palaniappan P, Dhingsa R, Lobo DN (2004) Recent developments in diagnosis of pancreatic cancer. Br Med J 329: 668–673
Tate AR, Martin AGR, Ali A, Cassell JA (2011) Using free text information to explore how and when GPs code a diagnosis of ovarian cancer: an observational study using primary care records of patients with ovarian cancer. Br Med J Open 102 (6): 947–951
Virlos I, Siriwardana H, Cemal Y, Siriwardena A (2005) Pathways of care for patients with suspected cancer of the pancreas: A tiered questionnaire-based survey of medical personnel across a single United Kingdom Calman-Hine cancer network. J pancreas 6: 13–25
Wilson H, Butler L, Repetto G, Love J (2000) Providing care to patients with pancreatic cancer: a retrospective chart review. Can Oncol Nurs J 10: 134–138
Acknowledgements
The authors would like to acknowledge the contribution to the research presented in this paper made by the Discovery Programme Steering Committee comprising: Roger Jones (chair); Jonathan Banks; Alison Clutterbuck; Jon Emery; Joanne Hartland; Sandra Hollinghurst; Maire Justice; Jenny Knowles; Helen Morris; Greg Rubin, members of Pancreatic Cancer UK and Pancreatic Cancer Research Fund. The study was funded from the NIHR Programme Grants for Applied Research funding scheme and approved by the Independent Scientific Advisory Committee (ISAC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Stapley, S., Peters, T., Neal, R. et al. The risk of pancreatic cancer in symptomatic patients in primary care: a large case–control study using electronic records. Br J Cancer 106, 1940–1944 (2012). https://doi.org/10.1038/bjc.2012.190
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.190
Keywords
This article is cited by
-
A taxonomy of early diagnosis research to guide study design and funding prioritisation
British Journal of Cancer (2023)
-
Online information analysis on pancreatic cancer in Korea using structural topic model
Scientific Reports (2022)
-
A systematic review of interventions to recognise, refer and diagnose patients with lung cancer symptoms
npj Primary Care Respiratory Medicine (2022)
-
Development, validation and effectiveness of diagnostic prediction tools for colorectal cancer in primary care: a systematic review
BMC Cancer (2020)
-
The association between unexpected weight loss and cancer diagnosis in primary care: a matched cohort analysis of 65,000 presentations
British Journal of Cancer (2020)