Abstract
Background:
A transcription regulatory complex (TRC) that includes Ets1, Ets2, PEA3 and β-catenin/T-cell factors regulates osteopontin (OPN) that is implicated in colorectal cancer (CRC) dissemination. The consistency of OPN transcriptional control between primary CRC and metastases is unclear. This study investigates expression and prognostic significance of the OPN–TRC in primary human CRC and associated colorectal liver metastases (CRLM).
Methods:
Osteopontin–TRC factors were assayed by digital microscopy in 38 primary CRCs and matched CRLM specimens and assessed against clinical prognosis.
Results:
In primary CRC, OPN expression intensity correlated with that of its co-activators, PEA3 (r=0.600; P<0.01), Ets1 (r=0.552; P<0.01), Ets2 (r=0.521; P<0.01) and had prognostic significance. Osteopontin intensity in primary CRC inversely correlated with the interval between diagnosis and resection of CRLM. Overall OPN intensity was lower in CRLM than primary CRC and correlations with co-activators were weaker, for example, Ets1 (P=0.047), PEA3 (P=0.022) or nonsignificant (Ets2). The ratio of OPN expression in CRLM vs primary CRC had prognostic significance.
Conclusion:
This study supports transcriptional control of OPN by known coregulators in both primary and secondary CRC. Weaker associations in CRLM suggest involvement of other unknown factors possibly from the liver microenvironment or resulting from additional genetic or epigenetic changes that drive tumour metastatic capability in OPN transcriptional control.
Similar content being viewed by others
Main
Successful establishment of metastases from circulating tumour cells requires integration of cell-autonomous oncogenic signalling with external cues from the specific tissue microenvironment of target organs (Fidler, 2002). Osteopontin (OPN) promotes invasiveness of colorectal cancer (CRC) (Irby et al, 2004) and other solid tumours (El-Tanani et al, 2006) and is a lead marker of human CRC progression (Agrawal et al, 2002; Eschrich et al, 2005). Osteopontin is regulated by Ets transcription factors partly by specific binding to the OPN promoter region (Sato et al, 1998) and partly through crosstalk with Wnt and other signalling pathways (El-Tanani et al, 2004). Combinatorial effects of Ets-1, Ets-2, PEA3 and β-catenin/T-cell factors (β-catenin/Tcfs) enhance OPN expression (El-Tanani et al, 2004) although unbound Tcf-4 may act as a transcriptional OPN repressor (El-Tanani et al, 2006). In primary CRC, molecular consequences of the initiating adenomatous polyposis coli mutation (Morin et al, 1997) include hyperactivation of β-catenin/Tcf signalling (Polakis, 2000) that upregulates PEA3 (Liu et al, 2004). This deregulated signalling may contribute to OPN overexpression (El-Tanani et al, 2004).
In addition to the molecular consequences of initiating mutations, the gene expression profiles of metastatic cells may be influenced by their supporting microenvironment in target organs (Nakamura et al, 2007). The consistency of the OPN-regulatory cassette between primary human CRC and related liver metastases, however, remains unclear. This issue has practical importance because surgery for liver metastases may be combined with a range of other therapeutic modalities (Garden et al, 2006) that could be guided by molecular profiling. Here, we assess expression and prognostic relevance of OPN-regulatory networks by immunohistochemistry and digital microscopy, in paired human primary CRCs and liver metastases.
Patients and methods
Patients
The study included 38 consecutive patients who underwent liver resection for colorectal liver metastases (CRLM) at the Mater Infirmorum Hospital Belfast between 1994 and 2003, for whom sufficient archive tissue was available. Clinicopathological characteristics were recorded including age, sex, site of colonic primary tumour, TNM classification and Duke's stage, date of colonic surgery, date of liver resection, number and segmental location of liver metastases, size of the largest liver metastasis, type of liver resection (left vs right, extended vs standard, anatomical vs non-anatomical atypical), resection margin status (R0 or other), immediate post-operative course (blood loss, blood transfusion, remnant liver function, extrahepatic organ failure, in-hospital mortality), details of adjuvant chemotherapy and date of death. Patients classified as Duke's D presented with synchronous metastases. Those with classification Duke's A to C developed metachronous metastases.
Ethical approval
This study was approved by the Northern Ireland Research Ethics Committee.
Immunohistochemistry
Archived formalin-fixed paraffin-embedded tissue blocks from the primary colon resection specimen and the matching resected CRLM were sectioned (5 μm), mounted on APS-coated slides and anonymised by a coding system that preserved the within-patient matched-pair design. Immunohistochemical staining was automated on a DAKO autostainer (DAKO, Cambridgeshire, UK) through standard dewaxing, blocking, staining and washing protocols. Antigen retrieval was performed by microwaving in citrate buffer at high power for 10 min. Consecutive sections were stained with antibodies (at 1:100 dilution) to the following proteins: OPN (MAb MBIII B10 mouse/hybridoma, DHSB, University of Iowa, USA); β-catenin (H-102, sc-7199, Santa Cruz Biotechnology, Santa Cruz, CA, USA); Tcf4 (H-125, sc-13027, Santa Cruz Biotechnology); Ets-1 (H-150, sc-22802, Santa Cruz Biotechnology); Ets-2 (H-140, sc-22803, Santa Cruz Biotechnology); and PEA3 (H-120, sc-22806, Santa Cruz Biotechnology) or negative control where no primary antibody was used. Secondary visualisation was performed for all sections (including the no primary antibody controls), using DAKO Envision Plus HRP Kits (K4007, DAKO) according to the manufacturer's instructions.
Digital assessment of gene expression and data analysis
Slides were scanned digitally using an Aperio Scanscope CS2 (Aperio Technologies Inc., Vista, CA, USA) at × 40 objective magnification. Each slide was scanned in its entirety and stored as a jpeg format compressed tiff (svs) file (approximately 0.5 Gb per slide). Slide images were viewed using ImageScope (Aperio Technologies Inc.) and annotated digitally by accurately tracing the area of interest corresponding to tumour tissue. The annotated area was segmented using pixel density threshold analysis developed in-house and run within the ImageScope software (Figure 1). This used custom parameters detailed in Supplementary Table S1. The number and staining intensity of each pixel within the area of interest was calculated and exported for analysis via Microsoft Excel. Positivity was defined as the number of pixels exceeding the set threshold for staining intensity divided by the total number of pixels within the annotated area, expressed as a proportion. Intensity of staining was a continuous scale variable defined as the sum of staining intensities of all pixels within the annotated area. Subsequent statistical analysis and comparison with clinicopathological data was performed using SPSS v14.0 (SPSS Inc., Chicago, IL, USA). The relationship of immunohistochemical covariates (expressed as the ratio of staining intensity between colon and liver), clinical and pathological features to survival after hepatic resection was investigated by a Cox proportionate hazard model.
Results
A total of 26 patients were male and 12 were female with median age of 62.1 years at colectomy (interquartile range, 12.8 years). Pre-operative liver synthetic function (albumin and prothrombin time) were within the normal reference range and all patients were seronegative for hepatitis B and C. Details of tumour primary site, Duke's classification and TNM stage are presented in Table 1. The median interval between colectomy for primary CRC and liver resection for metastatic disease was 1.51 years (95% CI: 0.84, 2.18 years). The site, number, size, resection margin of liver metastases are presented in Table 2. The median survival time from liver resection was 3.90 years (95% CI: 3.2, 4.6 years) and from colectomy was 4.95 years (95% CI: 4.05, 5.85 years). In all, 20% of patients remained free of detectable cancer at 5 years after liver resection.
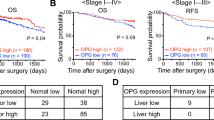
Expression of OPN and its transcriptional regulatory complex including Ets1, Ets2, PEA3, Tcf4 and β-catenin were detected by immunohistochemistry and digital scanning microscopy (Figures 1 and 2). Positive correlations between OPN intensity and that of PEA3 (r=0.600; P<0.01), Ets1 (r=0.552; P<0.01), Ets2 (r=0.521; P<0.01) were observed in primary tumours. Osteopontin positivity was generally lower in CRLM in comparison with that of the primary CRC (P<0.001; Figure 2, Table 3). The intensity of OPN expression in primary CRCs inversely correlated with the disease-free interval between colectomy and liver resection for metastases (Pearson's correlation coefficient, r=−0.123, P=0.045; Figure 3). Conversely, expression of each component of the OPN regulatory complex including Ets factors, Tcf4 and β-catenin was higher in CRLM than in primary tumours (Table 3). Weakly positive correlations were observed between OPN, Ets1 (P=0.047), PEA3 (P=0.022), Tcf4 (P=0.028) but not Ets2 in CRLM. In the majority of matched pairs, OPN expression was decreased or unchanged in the liver metastasis compared with the primary tumour, whereas Ets1, Ets2, PEA3, Tcf4 and β-catenin were predominantly increased (Table 4). The prognostic significance of differential expression of OPN and components of its regulatory complex between primary and secondary tumour was explored by Cox analysis. The ratio of OPN expression between paired samples of primary CRC and CRLM had prognostic significance after liver resection (Table 5).
Osteopontin and coregulators in primary CRC and CRLM matched pairs. Consecutive sections of colorectal primary tumour and liver metastasis, illustrating the raw scanned image and the positive pixel count for each primary antibody. Line diagrams show the difference between primary tumour and matched liver metastasis for each patient.
Discussion
Osteopontin is an important pro-metastasis gene with complex molecular regulation making its study in human primary and metastatic CRC a particular interest. Ets1, Ets2, PEA3 and Tcf factors bind to specific domains in the OPN promoter (El-Tanani et al, 2004, 2010). However, the identification of defined regulatory domains within the OPN promoter is insufficient evidence for involvement of single or combined transcriptional regulators in OPN-driven neoplastic progression. For example, PEA3 is a potent activator of pro-oncogenic OPN (El-Tanani et al, 2004) but may also inhibit breast cancer progression (Xing et al, 2000). Hence, interaction of coregulators with OPN may be context-specific.
Approximately 20–25% of CRC patients will have detectable liver secondaries at the time of the initial diagnosis and a further 40–50% of patients will typically develop hepatic metastases within 3 years of colectomy (Stangl et al, 1994). In this study, patients predominantly had Duke's C and D CRCs at presentation and had a median interval of 18 months between colectomy and liver resection. In this poor prognostic cohort, OPN and its coregulators including Ets factors, β-catenin and Tcf4 were detected in primary CRCs and liver metastases by digital microscopy. This method assesses the number of positive pixels as well as the intensity of immunohistochemical staining in annotated areas of histological tissue sections, using specific algorithms (Steinberg and Ali, 2001; Costello et al, 2003; Molnar et al, 2003). We manually annotated the area of interest corresponding to tumour tissue for digital microscopic assessment. This remains the most accurate method currently available and ensures that marker expression is predominantly confined to tumour cells. Although some background marker expression is inevitable in non-tumour cell fractions of primary and secondary CRC, such quantitative image analysis has superior reproducibility and consistency over observer scoring, in immunohistochemical assays (Ellis et al, 2005). By this method, we found that the intensity of OPN expression in primary CRCs, correlated directly with that of key OPN co-activators including PEA3, Ets1 and Ets2 and inversely correlated with the interval until liver resection for CRLM. These findings support prognostic relevance of OPN in CRC, as previously reported (Agrawal et al, 2002). Furthermore, our study provides the scientific foundation for further fundamental investigations in model systems, which may accelerate validation of OPN and/or coregulators as molecular targets for therapy.
Signals from an inhospitable tissue microenvironment may influence the growth of metastatic tumour (Fidler, 2002; Nakamura et al, 2007). In this study, expression of OPN co-activators including Ets1, Ets2, PEA3 and β-catenin were increased in the majority of CRLM vs primary CRC. However, these changes were not accompanied by any relative increase in OPN expression, which was unchanged in 21 patients and decreased in 17 CRLMs vs primary CRCs. Tcf4 is a potential suppressor of OPN when not complexed with β-catenin (El-Tanani et al, 2006) and was also increased in CRLM in comparison with primary CRC. However, no significant correlations were observed between Tcf4 and OPN in CRLM. It appears unlikely that Tcf4 alone would have overcome the combinatorial enhancing effects of Ets1, Ets2, PEA3 and β-catenin, on OPN expression in CRLM. Although correlations were observed between OPN expression and that of its co-activators in liver metastases, these were weaker than those of primary CRC. Cox analysis demonstrated that the ratio of OPN expression in the primary tumour to that in CRLM (colon to liver ratio) had prognostic significance.
Taken together, our study shows expression differences of OPN and its coregulators between primary CRC and liver metastases. The weaker correlations between OPN and its coregulators in CRLM suggest context specificity of OPN transcriptional control. Unknown signals from the organ microenvironment or additional genetic or epigenetic changes that accumulate in tumour cells as they acquire metastatic capability may also influence OPN regulation, implicated in progression of tumour growth.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ (2002) Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst 94: 513–521
Costello SS, Johnston DJ, Dervan PA, O’Shea DG (2003) Development and evaluation of the virtual pathology slide: a new tool in telepathology. J Med Internet Res 5: e11
El-Tanani M, Platt-Higgins A, Rudland PS, Campbell FC (2004) Ets gene PEA3 cooperates with beta-catenin-Lef-1 and c-Jun in regulation of osteopontin transcription. J Biol Chem 279: 20794–20806
El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS (2006) The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev 17: 463–474
El-Tanani MK, Jin D, Campbell FC, Johnston PG (2010) Interferon-induced transmembrane 3 binds osteopontin in vitro: expressed in vivo IFITM3 reduced OPN expression. Oncogene 29: 752–762
Ellis CM, Dyson MJ, Stephenson TJ, Maltby EL (2005) HER2 amplification status in breast cancer: a comparison between immunohistochemical staining and fluorescence in situ hybridisation using manual and automated quantitative image analysis scoring techniques. J Clin Pathol 58: 710–714
Eschrich S, Yang I, Bloom G, Kwong KY, Boulware D, Cantor A, Coppola D, Kruhoffer M, Aaltonen L, Orntoft TF, Quackenbush J, Yeatman TJ (2005) Molecular staging for survival prediction of colorectal cancer patients. J Clin Oncol 23: 3526–3535
Fidler IJ (2002) Critical determinants of metastasis. Semin Cancer Biol 12: 89–96
Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, Primrose JN, Parks RW (2006) Guidelines for resection of colorectal cancer liver metastases. Gut 55 (Suppl 3): iii1–iii8
Irby RB, McCarthy SM, Yeatman TJ (2004) Osteopontin regulates multiple functions contributing to human colon cancer development and progression. Clin Exp Metastasis 21: 515–523
Liu Y, Borchert GL, Phang JM (2004) Polyoma enhancer activator 3, an ets transcription factor, mediates the induction of cyclooxygenase-2 by nitric oxide in colorectal cancer cells. J Biol Chem 279: 18694–18700
Molnar B, Berczi L, Diczhazy C, Tagscherer A, Varga SV, Szende B, Tulassay Z (2003) Digital slide and virtual microscopy based routine and telepathology evaluation of routine gastrointestinal biopsy specimens. J Clin Pathol 56: 433–438
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275: 1787–1790
Nakamura T, Fidler IJ, Coombes KR (2007) Gene expression profile of metastatic human pancreatic cancer cells depends on the organ microenvironment. Cancer Res 67: 139–148
Polakis P (2000) Wnt signaling and cancer. Genes Develop 14: 1837–1851
Sato M, Morii E, Komori T, Kawahata H, Sugimoto M, Terai K, Shimizu H, Yasui T, Ogihara H, Yasui N, Ochi T, Kitamura Y, Ito Y, Nomura S (1998) Transcriptional regulation of osteopontin gene in vivo by PEBP2alphaA/CBFA1 and ETS1 in the skeletal tissues. Oncogene 17: 1517–1525
Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J (1994) Factors influencing the natural history of colorectal liver metastases. Lancet 343: 1405–1410
Steinberg DM, Ali SZ (2001) Application of virtual microscopy in clinical cytopathology. Diagnostic Cytopathol 25: 389–396
Xing X, Wang SC, Xia W, Zou Y, Shao R, Kwong KY, Yu Z, Zhang S, Miller S, Huang L, Hung MC (2000) The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat Med 6: 189–195
Acknowledgements
We are grateful to Hilary McBride, Immunohistology, Royal Victoria Hospital, Belfast, and Sid Trewin, Pathology, Queen's University of Belfast and for the unwavering support and mentorship of DJM by Professor OJ Garden (Clinical and Surgical Sciences) and Professor John P Iredale (MRC Centre for Inflammation Research), University of Edinburgh. This work was supported by a Cancer Research UK Research Training Bursary (C19075/A6164) to DJM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Mole, D., O'Neill, C., Hamilton, P. et al. Expression of osteopontin coregulators in primary colorectal cancer and associated liver metastases. Br J Cancer 104, 1007–1012 (2011). https://doi.org/10.1038/bjc.2011.33
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.33
Keywords
This article is cited by
-
Biological resonance for cancer metastasis, a new hypothesis based on comparisons between primary cancers and metastases
Cancer Microenvironment (2013)