Key Points
-
Discusses the causes and impact of dry mouth.
-
Discusses the management of dry mouth and Sjögren's.
-
Highlights those patients who should be referred to a specialist centre for management
Abstract
Oral dryness is a very common condition presenting to a general dental practitioner or hospital specialist. The most common cause of oral dryness is drug related, however, patients with Sjögren's syndrome, a multisystem autoimmune condition, may present to their dentist rather than their GP complaining of dry mouth and dry eyes. This update article explores the causes of oral dryness and how to manage it. The update on Sjögren's syndrome explains the latest relevant diagnostic criteria, presenting signs, symptoms, investigations and management principles.
Similar content being viewed by others
Introduction
Oral dryness, or xerostomia, is a common complaint affecting about 13% of the population in the UK.1 It may be the result of hypo-salivation or because of a change in salivary composition. The term subjective xerostomia can be used to describe the condition where a patient perceives a reduction in their oral mucosal wetness or complains of a lack of oral lubrication when there is normal salivary function present.
The normal average salivary flow rate is variable during the day with a person producing approximately 500 ml over 24 hours, but during eating it increases to about 2.0 ml/min, reducing at night to about 0.1 ml/min on average.
Dry mouth
Causes of dry mouth
There are many causes of dry mouth (Table 1),2 however, medication-related xerostomia is the most common cause.
Professor Andy Wolff and colleagues have published their systematic reviews of medication-induced salivary gland dysfunction recently.3,4,5,6 They have compiled a comprehensive list of medications with documented effects on salivary gland function or symptoms. They suggested that the list may also prove useful in helping practitioners anticipate adverse effects and consider alternative medications.3
The most commonly used medicines that cause a dry mouth include:
-
Antidepressants (tricyclic antidepressants, monoamine oxidases and selective serotonin reuptake inhibitors)
-
Antihistamines
-
Antihypertensives (ACE inhibitors, for example, ramipril, beta blockers [eg, atenolol, propranolol]).
-
Diuretics (bendroflumethiazide)
-
Anti-reflux (proton pump inhibitors eg, omeprazole)
-
Anti-cholinergics (atropine)
-
Benzodiazepines (diazepam)
-
Analgesics (opiates).
Wolff et al. discussed the pathogenesis of medication-induced salivary gland dysfunction methodology (MISGD) in their systematic review, concluding that the types of medications that were most commonly implicated for inducing salivary gland dysfunction were those acting on the nervous, cardiovascular, genitourinary, musculoskeletal, respiratory, and alimentary systems.4 In their review of the clinical implications of MISGD they commented on the significant burden of xerostomia and the negative impact on oral health.6 There is often a loose temporal relationship with the onset of symptoms after starting the medication, and with the dose of the drug. The sensation of a dry mouth usually resolves quite quickly once the medication is stopped, but it can persist. A single drug may have variable xerogenic effects across a population. The review confirmed that multiple medication use increased the risk of xerostomia as polypharmacy correlated strongly with xerostomia. There is also an association with age and developing MISGD, and so with an ageing population with ever increasing complex morbidities and polypharmacy, drug-induced xerostomia will increase. Dry mouth is not the only oral effect of medications; burning mouth, oral dysaesthesia and angioedema effects are well known.
Mouth breathing will cause the oral mucosa to dry out and many patients report their dryness is worst on waking, maybe because of mouth breathing at night. In these cases, there is usually no loss of salivary function, and so patients will not report problems during mealtimes or when speaking. Likewise, anxiety is another physiological cause of dry mouth without functional loss of saliva unless a patient is on an anxiolytic.
Irradiation for head and neck cancer, particularly HPV driven oropharyngeal carcinoma, is associated with considerable morbidity including severe oral dryness. More modern external beam radiation is salivary gland sparing. Patients who have had radioactive iodine for thyroid gland disease often have considerable hypo-salivation as iodine is selectively taken up in the salivary glands as well as by the thyroid gland. Salivary gland aplasia is a rare cause of oral dryness.
Impact of dry mouth
A patient with sparse or very thick saliva may have difficulty with swallowing, especially dry foods, and with speaking because their poorly lubricated tongue may adhere to the palate and likewise the lips may stick together. Patients who wear dentures will find that food and debris builds up around the dentures, their dentures may be difficult to control if there is poor suction due to an absence of saliva. Patients may also report an unpleasant taste and or halitosis, due to reduced oral clearance, secondary to a lack of saliva.
One of the most important consequences of hypo-salivation or altered saliva composition is an increase in caries. Surfaces not normally associated with caries in adults, ie smooth surfaces and particularly root surfaces, may be involved. Recurrent caries around restoration margins is common in patients with severe xerostomia (Fig. 1).
Assessment of dry mouth
When a patient presents either to a general dental practitioner or a hospital clinic, a thorough history is crucial for establishing any possible causes of dry mouth, followed by examination and relevant investigations, to reach a definitive diagnosis and to establish any underlying cause and whether there is reduced salivary secretion. The Challacombe dry mouth scale (Fig. 3) is a useful clinical aid for all dentists in assessing patients presenting with a dry mouth.7 The purpose of this scale is to be able to identify visually whether patients have signs of objective oral dryness and to quantify the severity. If the scale for an individual appears to change over time then this will help select the most appropriate interventions and therapy options. It works as an additive score of 1 to 10; 1 being the least and 10 being the most severe. Each feature scores 1 and symptoms will not necessarily progress in the order shown, however, summated scores indicate the likely need for treatment. Score changes over time can be used to monitor symptom progression or regression.
Xerostomia causes changes to the soft and hard tissues of the oral cavity recognisable by a GDP as well as specialist. Examination reveals many common features:
-
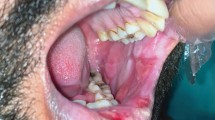
Instruments used to examine the oral cavity will stick to the buccal mucosa and tongue (Fig. 2)
Figure 4: Labial salivary gland biopsy. -
Saliva will be sparse or frothy and there maybe absence of saliva pooling in the floor of the mouth
-
Saliva expressed from the parotid gland ducts may be very sparse, particulate or even gelatinous (Fig. 8)
-
Due to the changes in saliva composition and volume, the saliva has a reduced buffering capacity, resulting in a lower pH and so patients are more susceptible to dental caries. In addition when there is a lack of saliva there are more cariogenic bacteria present (Streptococcus mutans and Lactobacilli) which also contributes to a susceptibility to dental caries. Caries mainly affects the cervical surfaces, cusp tips and incisal edges
-
The tongue may be lobulated, highly fissured, depapillated or atrophic
-
Debris may be present on the palate or under dentures
-
The gingiva architecture may be changed with lack of stippling and the oral mucosa may have a more shiny, glassy appearance
-
Patients who wear dentures may present with denture-induced stomatitis from an increase in Candida species and Staphylococci and this will appear as redness of the mucosa under the denture.
The relevant investigations include:
-
Sialometry – whole unstimulated flow rate is calculated by asking a patient to drool into a container over five minutes, rates of more than 0.2 ml/min are considered normal. Stimulated parotid flow can be measured via the application of 2% citric acid placed onto the postero-lateral aspects of the tongue and a collecting apparatus, or by asking a patient to chew paraffin wax to stimulate saliva. A rate of more than 0.4 ml/min is considered normal.
-
Blood tests to determine whether connective tissue associated antibodies (ENA, ANA, sDNA and rheumatoid factor) are present in association with a raised ESR or hyper-gammaglobulinaemia (raised IgG)
-
Labial gland biopsy has remained a gold standard investigation for diagnosing salivary gland disease. A labial gland biopsy is usually performed under local anaesthesia. A superficial incision is made on the labial mucosa of the lower lip (Fig. 4). Approximately six minor salivary glands are then collected and the incision is closed with resorbable sutures. The glands are assessed for the presence or absence of inflammation. Focal lymphocytic sialadenitis is characterised by the presence of one or more foci containing dense aggregates of 50 or more lymphocytes (that are usually located in perivascular or periductal locations). These foci are adjacent to normal-appearing mucous acini, in lobes or lobules that lack duct dilation and contain no more than a minority proportion of plasma cells. The number of foci in 4 mm2 gland is referred to as the focus score (semi quantitative assessment first described by Chisolm and Mason)8 and a focus score of more than 1 is diagnostic for Sjögren's syndrome (Fig. 5). Non-specific chronic sialadenitis is characterised by the presence of scattered or focal infiltrates of lymphocytes and macrophages that are not immediately adjacent to normal-appearing acini and located in gland lobules, lobes or entire glands that exhibit some combination of mild to moderate acinar atrophy, interstitial fibrosis and duct dilation, often filled with inspissated mucus (Fig. 6)
-
Ultrasound of major salivary glands. This investigation has become more common because in the hands of trained specialists it is becoming a very reliable, non-invasive, non-irradiating, inexpensive imaging alternative. Ultrasounds have not completely replaced sialography, as the latter remains the modality of choice if a salivary stone, rather than other salivary gland disease, is suspected. There is conflict in the published literature about the reliability of ultrasounds, due to bias, as suggested by a systematic review.9 However, there is growing recognition that the ultrasound score is a strong predictor of labial gland biopsy histopathology outcome10 and a recent consensus meeting of experts has identified echogenicity and homogeneity as the two most reliable items in ultrasound salivary gland features, to be used by ultrasound-salivary gland trained experts in the diagnosis of Sjögren's.11 (Fig. 7)
If a patient complains of dry eyes then investigations of the eyes are required as part of an assessment as to whether a patient has Sjögren's syndrome, the following tests are recommended.
Schirmer test – to determine lacrimal flow
A small strip of filter paper is placed inside the lower eyelid in both eyes and the patient is asked to close their eyes for five minutes. The paper is then removed and the amount of moisture is measured. Less than 5 mm wetting of the paper is indicative of dry eyes.
Ocular staining (lissamine green dye) – to determine extent of eye surface damage
A drop of lissamine green dye is placed in the eye and the patient is asked to blink twice to spread the stain over the conjunctiva and cornea. The staining is scored using a slit lamp (an optical instrument that provides a magnified image of the tear film and the ocular surface and allows examination of the anterior eye). The lissamine green dye stains any desiccated and dying cells on the ocular surface.
Tear break up time (TBUT) – to assess quality of tears
To measure TBUT, fluorescein is placed into the patient's tear film and the patient is asked not to blink while the tear film is observed using a slit lamp. The TBUT is recorded as the number of seconds that elapse between the last blink and the appearance of the first dry spot in the tear film.
These ocular staining and tear break up time tests are usually carried out by members of an ophthalmology team because they are familiar with the use of a slit lamp. A schirmer test can be undertaken by any of the dental team as long as they have had appropriate training.
Sjögren's syndrome
Sjögren's syndrome is a chronic systemic autoimmune disease characterised by focal lymphocytic inflammatory infiltrates within the exocrine glands, particularly salivary and lacrimal, resulting in dry eyes (xerophthalmia) and dry mouth (xerostomia). Involvement of the sweat and sebaceous glands manifest as very dry skin and involvement of mucous glands can result in a dry nose, throat and vagina. In addition, numerous extra-glandular features may develop:
-
Arthralgia
-
Arthritis
-
Raynaud's phenomenon Myalgia
-
Leukopenia and anaemia
-
Lymphadenopathy
-
Vasculitis
-
Pulmonary disease
-
Gastrointestinal disease
-
Neuropathy
-
Renal tubular acidosis
-
Lymphoma.
About 50% of patients with Sjögren's have cutaneous findings such as dry skin (xeroderma) palpable and non-palpable purpura and/or urticaria. Primary Sjögren's occurs in the absence of an underlying connective tissue and secondary Sjögren's occurs in the presence of a pre-existing connective tissue disease such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE). It is often difficult to determine whether the presenting symptoms are solely due to Sjögren's as there is considerable overlap between Sjögren's and these connective diseases.
Classification criteria
Multiple classification systems were devised between 1965 and 2002. The most commonly used criteria were the American-European Consensus Group (AECG) criteria.12 In 2012, new classification criteria were developed using the NIH-funded Sjögren's International Collaborative Clinical Alliance (SICCA) registry and were provisionally approved by the American College of Rheumatology (ACR).13 These criteria were designed to classify individuals being enrolled in clinical trials. The cohort recruited into the study for the development and validation of the criteria were patients who had signs and symptoms suggestive of Sjögren's. Subsequent analysis to compare the AECG and ACR criteria utilised a cohort of patients from the USA who had not been used in either the AECG or ACR criteria development and there was a high level of concordance. A consensus group from the ACR criteria team and EUALR Sjögren's Task Force have recently agreed on criteria which combine the ACR and AECG criteria.14
The consensus criteria recently published14 are based on the weighted sum of five items:
-
1
Anti-SSA/Ro antibody positivity – scores 3
-
2
Focal lymphocytic sialadenitis with a focus score of ≥1 foci/4 mm2 – scores 3
-
3
An abnormal ocular staining score of ≥5 (or van Bijsterveld score of ≥4) – scores 1
-
4
Schirmer's test result of ≤5 mm/5 min – scores 1
-
5
Unstimulated salivary flow rate of ≤0.1 ml/min – scores 1.
Individuals with signs and/or symptoms suggestive of SS who have a total score of ≥4 for the above items meet the criteria for primary Sjögren's.
Sensitivity and specificity against clinician-expert-derived case/non-case status in the final validation cohort were high, that is, 96% (95% CI 92% to 98%) and 95% (95% CI 92% to 97%), respectively.14
Aetiopathogenesis of Sjögren's
The aetiology of Sjögren's is not well understood. It is thought to be multifactorial in the presence of a predisposing genetic background with environmental triggers such as viruses. The presence of activated salivary gland epithelial cells expressing major histocompatibility complex (MHC) class II molecules and the identification of inherited susceptibility markers suggest that environmental or endogenous antigens trigger a self-perpetuating inflammatory response in susceptible individuals. Hyper-reactive polyclonal B lymphocytes produce various auto-antibodies in this syndrome, particularly Ro and La (SSA and SSB), in greater than 75% of cases. In addition, the continuing presence of active interferon pathways in Sjögren's syndrome suggests ongoing activation of the innate immune system. Overexpression of several cytokines has been demonstrated including TNFa, IL-6, IL-1, IL-18 and IL-22. Likewise, CXCL13 and other chemokines along with B cell pathway factors such as BAFF and April have been extensively studied in Sjögren's to better understand epithelial cell and lymphocyte interactions and to provide targets for therapy.15
Epidemiology and prognosis of Sjögren's
Sjögren's is thought to be the second most common rheumatologic disorder after SLE in the USA with about 0.1–4% of the population affected. International comparative studies between different ethnic groups has suggested that it is a homogeneous disease that has a similar prevalence in differing countries with an estimated 1–2 million people affected. The male to female ratio is 9:1 affecting individuals of any age, but it is more common in post-menopausal women. The prognosis is generally good although a small cohort developing the most serious complications such as cryoglobulinaemia or lymphoproliferative disease have a poorer prognosis. Morbidity is mainly associated with the loss of function of the exocrine glands, and debilitating fatigue. Patients with Sjögren's are at a much higher risk of developing non-Hodgkin lymphoma than the normal population; often quoted as up to 20 times higher than in the general population. The most common type of lymphoma is mucosa associated lymphoid tissue (MALT) lymphoma. These lymphomas are low grade and have a very slow progression. Germinal centre formation seen within the minor salivary glands are thought to be predictive of progression to MALT lymphoma.16 Other known risk factors include generalised lymphadenopathy, leukopenia, particularly low CD4 counts, hypergammaglobulinaemia and cryoglobulinaemia. Women who have positive antibodies against SSA/Ro antibodies have a 2% risk of having a child born with congenital heart block, but for those who have already had a child with heart block the risk is 15%.
Management of oral dryness
The main aims for treatment of oral dryness are to relieve the symptoms of dryness by replacing or stimulating saliva production, to restore function and to prevent further damage of soft and hard tissues of the oral cavity. Patients are advised to take regular sips of water throughout the day. Artificial saliva sprays and lozenges can provide temporary replacement of saliva and can be used throughout the day to provide effective oral lubrication. The patient's own saliva can be stimulated using sugar free chewing gum, sucking sugar-free mints, local sialagogues such as saliva stimulating tablets, and systemic sialagogues pilocarpine or cevimeline. The latter two agents have been recommended as a first line treatment for radiotherapy-induced xerostomia.17
Patients should be advised to have a diet low in refined carbohydrates and to avoid acidic snacks and drinks. Fluoride toothpastes (Duraphat 500 ppm), gels and alcohol-free fluoride rinses can be used daily by the patient for the prevention of dental caries. Duraphat varnish can be professionally applied by the dental team on an intermittent basis. In addition, it is important to control infection of the oral cavity. Regular intermittent courses (one week in four) of topical antifungals can be prescribed and the use of antibacterial mouthwashes is recommended.
Devices delivering artificial saliva and gels into the mouth via two-part dentures with reservoirs and soft polyacrylic bite raisers with chambers either buccal to the lower arch or the palate of an upper device have been tried.18 However, these devices are bulky, expensive to create and only available through specialist centres. Electrostimulation devices have been available for some time but were not widely available until recently. Trials are under way to assess a device that has become commercially available in the UK.
Management of Sjögren's
The principles of management for Sjögren's encompass management of the salivary, ocular and systemic extra glandular components. Consequently, the principles of management of oral dryness outlined above pertain to the management of a patient with Sjögren's. The ocular component is similar in that artificial tears and stimulants such as topical pilocarpine are available for the management of dry eye. Preservative free eye drops are often less of an irritant and there are many preparations available to patients via advice from high street opticians. Patients with very severe dry eye should be referred to an ophthalmology clinic either via their optician or their GP.
Systemic manifestations
Whilst no disease-modifying antirheumatic drugs (DMARDs) have been shown in randomised, placebo-controlled trials to be effective for the treatment of primary Sjögren's syndrome, hydroxychloroquine has been used widely for reducing the symptoms of fatigue and joint pain in primary Sjögren's syndrome. However, it failed in a randomised controlled trial to significantly reduce symptoms of dryness, pain, and fatigue over placebo.19 Methotrexate has not been well studied for the treatment of primary Sjögren's syndrome. More recently, the tumour necrosis factor-α inhibitors infliximab and etanercept have been investigated for their efficacy and safety in patients with primary Sjögren's syndrome and neither was found to be effective for improving disease outcomes.20,21 Rituximab an anti-CD20 antibody which depletes B cells has been evaluated in several trials with mixed results. The use of biologics in Sjögren's has been reviewed recently.22 The UK study Tractiss which recruited 133 Ro positive Sjögren's patients with fatigue and oral dryness indicated that Rituximab is neither clinically effective nor cost-effective in the recruited patient population.23 The management of fatigue, which is often very debilitating for patients, is difficult, however, evidence suggests that a multidisciplinary approach including physiotherapy and psychology is beneficial.24
Summary
Oral dryness and Sjögren's syndrome are common debilitating conditions, the latter primarily affecting post-menopausal women. Xerostomia secondary to medications is the most common cause of oral dryness which can improve on withdrawal of the associated medicines. The benefit of biologics in Sjögren's has yet to be proven and at present there is no single effective treatment for the disease. Fatigue is very often troublesome for patients with Sjögren's syndrome and requires a multidisciplinary management approach. MALT lymphoma is a serious complication of Sjögren's and so any patient with swelling of the salivary glands should be referred to a specialist centre for management of their disease.
References
Field E A, Fear S, Higham S M et al. Age and medication are significant risk factors for xerostomia in an English population, attending general dental practice. Gerodontology 2001; 18: 21–24.
Scully C, Felix D H . Oral Medicine — Update for the dental practitioner. Dry mouth and disorders of salivation Br Dent J 2005; 199: 423–427.
Wolff A, Joshi R K, Ekstrom J et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R D 2017; 17: 1–28.
Villa A, Wolff A, Narayana N et al. World Workshop on Oral Medicine VI: a systematic review of medication-induced salivary gland dysfunction. Oral Dis 2016; 22: 365–382.
Villa A, Wolff A, Afraiman D et al. World Workshop on Oral Medicine VI: a systematic review of medication-induced salivary gland dysfunction: prevalence, diagnosis, and treatment. Clin Oral Invest 2015; 19: 1563–1580.
Aliko A, Wolff A, Dawes C et al. World Workshop on Oral Medicine VI: clinical implications of medication-induced salivary gland dysfunction Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120: 185–206.
Challacombe S . The Challacombe Scale. Available at www.challacombescale.co.uk (accessed October 2017).
Chisholm D M, Mason D K . Labial salivary gland biopsy in Sjögren's Syndrome J Clin Pathol 1968; 21: 656–660.
Delli K, Dijkstra P U, Stell A J . Diagnostic properties of ultrasound of major salivary glands in Sjögren's syndrome: a meta-analysis Oral Dis 2015; 21: 792–800.
Astorri E, Sutcliffe N, Richards P et al. Ultrasound of the salivary glands is a strong predictor of labial gland biopsy histopathology in patients with sicca symptoms. J Oral Pathol Med 2016; 45: 450–454.
Jousse-Joulin S, Nowak E, Cornec D et al. Salivary gland ultrasound abnormalities in primary Sjögren's syndrome: consensual US-SG core items definition and reliability RMD Open 2017; 3: e000364.
Vitali C, Bombardieri S, Jonsson R et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61: 554–558.
Shiboski S C, Shiboski C H, Criswell L A et al. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012; 64: 475–487.
Shiboski C H, Shiboski S C, Seror R et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome; A consensus and data-driven methodology Involving three International patient cohorts. Ann Rheum Dis 2017; 76: 9–16.
Barone F, Colafrancesco S . Sjögren's syndrome: from pathogenesis to novel therapeutic targets Clin Exp Rheumatol 2016; 34 (Suppl. 98): S58–S62.
Theander E, Vasaitis L, Baecklund E et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann Rheum Dis 2011; 70: 1363–1368.
Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S . Interventions for the management of radiotherapy-induced xerostomia and hypo salivation: A systematic review and meta-analysis. Oral Oncol 2017; 66: 64–74.
Frost P M, Shirlaw P J, Challacombe S J, Fernandes-Naglik L, Walter J D, Ide M . Impact of wearing an intra-oral lubricating device on oral health in dry mouth patients. Oral Dis 2006; 12: 57–62.
Gottenberg J E, Ravaud P, Puéchal X et al.: Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren's syndrome: the JOQUER randomized clinical trial. JAMA 2014; 312: 249–258.
Mariette X, Ravaud P, Steinfeld S et al.: Inefficacy of infliximab in primary Sjögren's syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren's Syndrome (TRIPSS). Arthritis Rheum 2004; 50: 1270–1276.
Moutsopoulos N M, Katsifis G E, Angelov N et al.: Lack of efficacy of etanercept in Sjogren syndrome correlates with failed suppression of tumour necrosis factor alpha and systemic immune activation. Ann Rheum Dis 2008; 67: 1437–1443.
Nocturne G, Cornec D, Seror R, Mariette X . Use of Biologics in Sjögren's Syndrome. Rheum Dis Clin North Am 2016; 42: 407–417
Bowman S J, Everett CC, O'Dwyer J L et al. Randomized Controlled Trial of Rituximab and Cost-Effectiveness Analysis in Treating Fatigue and Oral Dryness in Primary Sjögren's Syndrome. Arthritis Rheumatol 2017; 69: 1440–1450.
Hackett K L, Deane K H O, Strassheim V A et al. Systematic review of non-pharmacological interventions for primary Sjögren's syndrome. Rheumatology 2015; 54: 2025–2032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Shirlaw, P., Khan, A. Oral dryness and Sjögren's: an update. Br Dent J 223, 649–654 (2017). https://doi.org/10.1038/sj.bdj.2017.882
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2017.882