Key Points
-
Presents a comprehensive overview of common craniofacial malformations with a focus on orthodontic management.
-
This article is aimed at general dental practitioners and is supported with evidence where applicable.
Abstract
This review article presents an overview of craniofacial malformations and the role of the orthodontist in their management. The first part of this article focuses on cleft lip and palate, followed by more complex deformities including craniosynostosis and craniofacial microsomia. The main features of these anomalies are discussed as well as the clinical problems seen in this group of patients. The emphasis is on the role of the orthodontist in the multi-disciplinary management of these cases.
Similar content being viewed by others
Introduction
The aim of this paper is to describe the role of the orthodontist in the management of patients with cleft lip and palate (CLP) and complex craniofacial deformities.
Cleft lip and palate
Cleft lip and/or palate (CLP) is the most common craniofacial abnormality, occurring in approximately 1:700 live births.1 The incidence of cleft palate (CP) occurring alone is about 1:2,000 live births.2 In Caucasian populations, cleft lip (CL) with or without CP occurs more frequently in males than females by about 2:1 but CP alone occurs more frequently in females than males by about 2:1.3 Broadly speaking, about 70% of CLP cases are non-syndromic, occurring as an isolated condition, while the remaining 30% are associated with syndromes.4 Over 300 syndromes are known to have clefting of the lip or palate as an associated feature. These include syndromes such as van der Woude, Ectodermal dysplasia, Pierre Robin sequence, holoprosencephaly, Treacher Collins and Stickler syndromes.
The management of a patient with a cleft is carried out by a multi-disciplinary team of healthcare professionals who undertake care from birth through to adulthood. The core members of the team are the cleft surgeons (usually a combination of oral and maxillofacial and or plastic surgeons) orthodontist, speech and language therapist, and ENT surgeons.
Aetiology of CLP
The aetiology of both CL with or without CP is multifactorial with both genetic and environmental factors involved.4 The sibling risk for CLP is approximately 30 times higher than that for the normal population prevalence, while the concordance rate in monozygotic twins is approximately 25-45% as opposed to 3-6% for dizygotic twins.5 This lack of complete concordance, however, illustrates the importance of environmental factors in the aetiology of this condition. A growing number of mutant mouse strains that exhibit CP and to lesser extent CLP have now been developed and these continue to provide a host of candidate genes for CLP.6 Gene mutation in interferon regulatory factor 6 (IRF-6) gene has been implicated in clefting in van der Woude syndrome7 and the polio virus receptor related 1 gene (PVRL1) gene as being responsible for an autosomal recessive ectodermal dysplasia associated with clefting.8
Epidemiological data suggest that environmental risk factors such as maternal smoking and alcohol consumption, poor nutrition and viral infection can be linked to CLP.9 Maternal smoking during pregnancy has been linked consistently with increased risk of both CL with or without CP and isolated CP.10 The role of alcohol in isolated orofacial clefts is less certain with positive associations reported in some studies but not others.11 Findings of observational studies suggest a role for maternal nutrition in orofacial clefts. In most studies, maternal use of multivitamin supplements in early pregnancy has been linked to decreased risk of orofacial clefts;12 in a meta-analysis multivitamin use during pregnancy was associated with a 25% reduction in birth prevalence of orofacial clefts.13
The LAHSAL code
The LAHSAL code splits the relevant parts of the mouth into six parts (Fig. 1):
-
Right lip
-
Right alveolus
-
Hard palate
-
Soft palate
-
Left alveolus
-
Left lip.
The LAHSAL code indicates for each part whether there is a complete cleft (upper case letter, for example, H), an incomplete cleft (lower case letter, for example, h) or no cleft.
Clinical Standards Advisory Group Study (CSAG)
In the late 1980s, some concern was raised among healthcare professionals regarding the quality of care being provided for children born with CLP or CP in the UK.16,17 This was based principally upon the outcome of two studies:
The GOSLON Yardstick (Great Ormond Street, London and Oslo) was developed as a clinical tool that categorised dental arch relationships into five discrete categories. Using this yardstick, comparison between UK and Norwegian cleft centres demonstrated significant shortcomings in outcomes associated with the UK centre.17,18
Eurocleft - a European, multicentre clinical audit of treatment outcome for complete unilateral CLP. It found the two UK centres that participated to be weakest on almost every aspect of care.16
In 1995 the Department of Health in the UK charged the Clinical Standards Advisory Group to investigate the quality of care within the UK. All children in the UK with unilateral complete CLP aged 5 or 12 years of age in 1996-1997 were examined. Their speech, hearing, appearance, dental malocclusion, dental health, quality of bone graft (12 years) and skeletal base relationships were examined. The study found that the average result in all these areas was poor.19 Fifty-seven centres provided cleft care with a paucity of good clinical records. The volume of surgery carried out by each surgeon was variable, with an average of six cases per year.20
The CSAG made several recommendations,21 including:
-
1
Expertise should be limited to 6-15 centres in the UK
-
2
Each centre should provide a full range of cleft care
-
3
Documentation must be improved, including a nationwide database
-
4
Results should be regularly audited allowing comparisons between centres
-
5
Training should be provided for specialists in cleft care in high volume centres only.
On the basis of this investigation, the clinical management of children born with clefts is now carried out centrally in 11 centres. The cleft team at these centres includes a number of key members (Table 1), in addition to other specialists who may be involved with long-term care.
Problems in CLP
Feeding
A baby born with cleft lip and palate may experience difficulty in feeding at birth. Young noted that for 95% of parents, issues around feeding were the main concern.22
As a result of the CSAG report, all infants born with a cleft must have a feeding assessment within 24 hours of birth. Suckling can be slow because the baby can have difficulty generating adequate intraoral pressure and milk can be lost through the nose before it is swallowed. Added to this is the stabilising effect of the lips. Without the seal the lips should make around the teat, the baby struggles to keep the nipple in the correct position.23
Speech
Children with CLP can experience speech problems due to velopharyngeal insufficiency (VPI). VPI is the result of an inadequately functioning soft palate, which may be unable to lift and produce a good seal with the posterior pharyngeal wall. VPI can produce nasal escape on pressure consonants that is, k, p, t and is expressed as hypernasality.24 Spreistersbach et al.25 quoted 50% of children with repaired cleft palate developed normal speech spontaneously; 25% required speech and language therapy and 25% required further palatal surgery.
As a key member of the multidisciplinary team it is the role of the specialist speech and language therapist to monitor and assess speech and language development from birth to completion of treatment (approximately 20 years of age).
Hearing
There is a well-recognised association between CP and middle ear disease that is related to failure of the ventilatory function of the Eustachian tube. Eustachian tube dysfunction can be associated with otitis media with effusion (OME), commonly known as glue ear. The management of OME involves the use of ventilation tubes inserted through the tympanic membrane under general anaesthetic. In some centres ventilation tubes are inserted early and often repeatedly. In others a watch and wait policy is adopted. Unfortunately the evidence to support either view is not conclusive since repeated ventilation of the ear can result in similar problems to untreated OME.26
Oral and dental anomalies in CLP
Most children with CLP show a deficiency of soft tissue, reduced alveolar bone support, deficient sagittal maxillary growth, transverse collapse of the maxilla and a vertically short midface.27 Dental traits such as hypodontia, supernumerary teeth, peg-shaped teeth, crown and root malformations, dental asymmetry, midline deviation and delay in tooth development occur more commonly in children with cleft lip and palate.28,29
The developmental absence of the cleft side permanent lateral incisor is the most common finding in children with CLP (approximately 50% of patients with UCLP and BCLP). There is also an increased prevalence of hypodontia outside the cleft area in patients with CLP. Olin30 found a 24% prevalence of hypodontia outside the cleft area. Dewinter et al.31 reported the percentage of missing second maxillary premolars outside the cleft area in 22.2% of patients. The high prevalence of developmentally absent teeth in patients with clefts suggests a possible association between tooth agenesis and the cleft defect. It is known that genes that cause the cleft affect several tissues, including the dental lamina.6 The occurrence of cleft and hypodontia seems to be controlled by MSX-1 genes.32
The presence of a supernumerary tooth in the cleft region has been stated to be the second most common dental anomaly. Primary supernumerary teeth occur more commonly than permanent supernumeraries in the cleft region.33 The prevalence of supernumerary lateral incisors in the cleft area of 7.3% in patients with unilateral CLP and 6.7% in patients with bilateral CLP. A higher rate, 22.2%, of supernumerary permanent teeth in the cleft area was observed in children with a unilateral cleft lip or palate, or both.
Delayed loss of the primary teeth is commonly observed. Dental rotations most commonly affect the maxillary central and lateral incisors.
In the general population ectopic eruption of teeth has been reported to depend on systemic or local factors.34 The population average for ectopic teeth ranges between 2-6% for the maxillary first molars and 1.5-2% for the permanent canines.35 In a radiographic study of 225 children, a higher prevalence of ectopic eruption of the maxillary first permanent molar (15.4%) was found in children with a CL or CL and alveolus.36 Akcam et al.37 showed a significantly higher rate of impaction of maxillary canines in the anterior and premolar regions in the CLP group, with the highest rates in the anterior region on the cleft side.
Enamel hypoplasia of teeth in both primary and permanent dentitions is more common in CLP cases.38 Lucas et al.39 reported a higher prevalence of enamel discoloration in children with CLP compared with a control group and attributed this defect to trauma at the time of CLP surgery.
Studies on the overall dental health status of children born with clefts have been inconsistent. Dahllof et al.40 found that the frequency of caries and carious activity was significantly higher in the cleft group compared to the non-cleft group. This could not be explained by factors such as diet, fluoride exposure and irregularity of teeth.41 The authors hypothesised that this difference could be explained by parents of children with clefts having a more permissive attitude towards diet and between meal snacks, possibly in an attempt to compensate for the anatomical defect. A systematic review of caries prevalence in children with CLP found no firm evidence to support the view that children with CLP have an increased prevalence of caries.42
Numerous investigations show that the facial morphology in infants, children, adolescents and adults with CLP deviates from the norm.43 Hyashi et al.44 found that in CLP patients, the maxilla was smaller and located in a more posterior and upward position, upper facial height was less compared to the lower face and both upper and lower incisors showed marked lingual inclination. Rahman et al.45 also found that CLP children tended to have more Class III facial profiles due to maxillary hypoplasia. There is a suggestion that the maxillary hypoplasia is an intrinsic primary cause of the Class III skeletal pattern although Mars et al. believed that maxillary retrusion and abnormal maxillary growth is secondary to scarring caused by surgical repair.46
Management of CLP
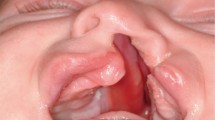
A child born with orofacial clefting will require complex long-term treatment, depending upon the severity of the cleft. The principle objectives of treatment are to establish a good facial appearance, good orofacial function during speech, eating and swallowing, an aesthetic, functional and stable occlusion and good hearing. As an example Figure 2 shows the results of lip repair in a young child with UCLP.
The treatment outline for the cleft patient from pre-birth onwards is summarised in Table 2.
Role of the orthodontist
In the neonatal period the parents and baby will usually be seen by the orthodontist, who will advise as to the immediate and longer term treatment that will be required. Presurgical orthopaedic plates have previously been recommended to help approximate the two segments of the maxilla over a period of 3-4 months. This is said to make primary palate and lip repair easier in both UCLP and BCLP cases. However, it is questionable whether repair is made much easier, and certainly there are no measurable long-term benefits from their use on dental arch relationships and facial growth.16 In the case of BCLP babies in particular, strapping is sometimes used, with or without a pre surgical orthopaedic plate, to help realign the premaxilla before surgical repair. Figure 3 shows the results of lip repair in a patient with BCLP.
As the upper central incisors erupt, it is not unusual for them to do so in cross bite with the lower incisors. Provided the skeletal pattern is favourable and the upper incisors are retroclined, an upper removable appliance, with posterior biteplanes to free the anterior occlusion, can be used to procline the upper incisors over the bite. Treatment usually takes between 3-6 months.
In the case of unilateral and bilateral complete CLP cases, orthodontic treatment is performed between the ages of 11-18 years to facilitate the provision of a bone graft in the alveolar cleft. An upper fixed appliance is often used along with a quadhelix. This is a fixed spring device with four helices seated above the palatal mucosa. A trihelix is useful if the interpremolar width is particularly narrow. The aim is to expand and round out the upper arch and create space for the alveolar bone graft at the cleft site (Fig. 4). The graft itself is taken from the iliac crest, skull, mandible or fibula. The bone graft should be undertaken before the eruption of the permanent canine to allow it to erupt through the bone graft (Fig. 5).
Once the permanent canines and premolars have erupted, the definitive phase of orthodontics, involving upper and lower fixed appliances, may begin. Treatment planning is carried out in conjunction with a restorative specialist to decide on space management when teeth are absent. If the cleft patient has a skeletal discrepancy that would benefit from orthognathic surgery, fixed appliance therapy is usually delayed until the patient is 16 years of age. Pre surgical orthodontics is usually carried out at this stage to align, level, decompensate and allow coordination of the arches (Fig. 6). This is usually followed by a Le Fort 1 maxillary advancement procedure to correct the underlying skeletal deformity.
In addition to these key stages the orthodontist is also responsible for record taking at five, ten, 15 and 20 years of age for national audit purposes.
Craniosynostosis
Craniosynostosis is the result of premature fusion of one or more skull sutures. It is rare with an overall incidence of 1:2000 – 1:2500 live births. When not associated with any birth defects it is referred to as non-syndromic and accounts for the majority (80+%). The head shape will be altered and the management is surgical with the aim of producing as close to as normal a head shape as possible. Functional problems for example, raised intracranial pressure, are rare in this group of patients. Patients, post-operatively, are followed up to keep an eye on their neuro-development and speech/language.
The more complex cases are classified as syndromic craniosynostosis. There are over 150 syndromes associated with craniosynostosis, the most common being Crouzon and Pfeiffer (one in 60,000 live births), Muenke (one in 140,000 live births) and Apert (one in 160,000 live births).48 The clinical features associated with these syndromes differ but include cranial vault deformities, midface hypoplasia/retrusion, malocclusion, cleft palate and hard/soft tissues hand and foot syndactyly.49 The patient illustrated in Figure 7 has Apert syndrome which is characterised by: bicoronal craniosynostosis, midface retrusion/hypoplasia, exorbitism and complex syndactyly of both the hands and feet.
Approximately 50% of patients with syndromic craniosynostosis are found to have a new mutation. Studies have identified mutations in genes such as FGFR1, FGFR2, FGFR3, MSX2, TWIST1, EFnB1 and TCF12. It is important for both patients and parents to be aware that most genetically determined craniosynostosis is characterised by an autosomal dominant inheritance pattern that is, an abnormal gene from one parent can cause the syndrome even though the matching gene from the other parent is normal – the abnormal gene dominates.50
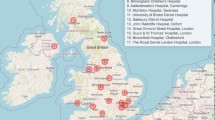
The clinical management from birth to maturity is undertaken by a dedicated interdisciplinary team which includes neuro-, plastic, oral and maxillofacial surgery, ENT, ophthalmology, dentistry, orthodontics, neuroradiology, clinical and molecular genetics, speech/language therapy, psychology and nursing.51 The management in most centres initially focuses on functional rehabilitation and this may include the surgical correction of the craniofacial deformity and involve multiple procedures with the final correction not accomplished until skeletal maturity. In England, patients with syndromic craniosynostosis are managed in four nationally commissioned craniofacial centres. These are based in Birmingham (Birmingham's Children's Hospital), Liverpool (Alder Hey Children's Hospital), London (Great Ormond Street Hospital for Children), and Oxford (John Radcliffe Hospital). The services are classified as highly specialised services and reflect the fact there are fewer than 500 new cases per year – this is in effect centralisation of services.52
What sort of functional problems can these patients run into?
-
Raised intracranial pressure, which can lead to problems with neuro-development and vision This is usually managed with some sort of cranial vault expansion
-
Eye exposure which can cause corneal abrasions and scarring
-
Compromised airway which may require an intervention from for example, nasal prongs, CPAP or in severe cases tracheostomy
-
Feeding.
Craniofacial Surgery
For patients with syndromic craniosynostosis the types of surgical procedure that are used to correct the skeletal deformity can be broadly divided into the following:
-
Posterior vault expansion
-
Fronto-orbital advancement (FOAR) and remodeling followed by the Le Fort III midface advancement (Fig. 8)
Figure 8: The Le Fort III osteotomy being used to advance the midface in a patient with Crouzon syndrome. In this case full orthodontic pre-surgical preparation with fixed appliances was carried out. Taken from chapter 19, 'Surgery' by Barry Jones, David Dunaway and Richard Hayward in 'The clinical management of craniosynostosis' edited by Richard Hayward, Barry Jones, David Dunaway and Robert Evans, 2004, ISBN 1-898683-36-0. Reproduced with kind permission of Mac Keith Press, www.mackeith.co.uk
-
Frontofacial advance (FFA - also referred to as the monobloc) ± a facial bipartition (FB). (Figs 9,10).
Figure 9: The frontofacial advancement (FFA). This is also referred to as the monobloc advance and advances the forehead, orbits and maxilla. The CT scans show evidence of a previous posterior vault expansion and the result of the FFA using external distraction. Taken from chapter 19, 'Surgery' by Barry Jones, David Dunaway and Richard Hayward in 'The clinical management of craniosynostosis' edited by Richard Hayward, Barry Jones, David Dunaway and Robert Evans, 2004, ISBN 1-898683-36-0. Reproduced with kind permission of Mac Keith Press, www.mackeith.co.uk
Figure 10: The facial bipartition. Moving the orbits into the correct position results in a transverse expansion of upper dental arch and creates a midline diastema the size of which is determined by the amount of orbital movement and the point of rotation. The intra-operative picture shows the orbits being located in their new position with a plate. Taken from chapter 19, 'Surgery' by Barry Jones, David Dunaway and Richard Hayward in 'The clinical management of craniosynostosis' edited by Richard Hayward, Barry Jones, David Dunaway and Robert Evans, 2004, ISBN 1-898683-36-0. Reproduced with kind permission of Mac Keith Press, www.mackeith.co.uk
The FOAR advances the forehead and the upper part of the orbits in two pieces. The Le Fort III osteotomy was originally described by Gillies in 1952.53 It effectively mobilises the facial skeleton from the skull base and includes the lower part of the orbits and the maxilla. The FFA was initially described by Ortiz-Monasterio et al. in 1978.54 The procedure advances the forehead, orbits and maxilla (including the maxillary dentition) in one piece – hence the term monobloc. The FB, initially described by van der Meulen in 197955 was later refined by Tessier.56 A midline facial split is created as an extension of the FFA, which allows the two 'hemifaces' to be rotated together narrowing the space between the orbits and expanding the maxilla. In addition the lateral aspects of the orbits can be moved posteriorly and the medial aspects anteriorly to correct the central facial concavity seen in Apert syndrome.
Distraction
One of the most significant changes in craniofacial surgical practice has been the introduction of distraction osteogenesis (DO). DO increases the length/volume of a bone by gradual separation of the bony segments. The enveloping soft tissues are also distracted thereby increasing their volume. The rate of distraction is generally 1 mm per day and the patient or parent turns the device for the desired period -anything between 10-14 days.
One major advantage of this technique includes removing the need for a bone graft and being able to achieve more lengthening/volume that is possible with conventional surgery. The technique was popularised by Evans in the 1940s in the management of complex leg injuries52,57,58 (Fig. 11). McCarthy and colleagues in the 1990s introduced DO to craniofacial surgical practice to lengthen the mandible in patients with hemifacial microsomia (Fig. 12).59 Subsequently, just about all regions of the craniofacial skeleton have been distracted with varying degrees of success using internal, external or a combination of both types of distractor.60,61 With regard to the skeletal deformity seen in Crouzon and Apert syndrome the use of distraction with FFA, FB and the Le Fort III procedures has become accepted practice with good results (Fig. 13).62
Complications
Major craniofacial surgery is not without risk. For the FFA mortality rates as high as 4.5% have been reported although this has reduced to 1% in more recent reports. With regard to morbidity the most concerning problems are cerebrospinal fluid leaks (incidence of 2-20%), major blood loss (greater than one blood volume 5.3 to 9.1%) and frontal bone necrosis requiring debridement and further cranioplasty (3-20%).63 Dental complications occur and include disruption to normal tooth development and eruption in the posterior maxilla after early pterygomaxillary dysjunction64 and damage to individual teeth following inter-dental osteotomies including ankylosis.
The role of the orthodontist
The role of the orthodontist will vary from team to team and from country to country. The preferred option is where the orthodontist is a core member of the team involved with much of the decision making and planning.65 The specific role of the orthodontist can be divided into three areas:
Coordinate dental care across primary and secondary care as needed
With so many medical and social issues to deal with it is easy for patients not to be in receipt of regular dental care and historically caries levels were higher than average frequently requiring treatment under GA including extractions.66,67,68,69 Close liaison with colleagues in the primary and secondary sectors is essential but requires coordination and ideally shared record keeping. In a histological study of extracted teeth from patients with Apert syndrome identified histological anomalies of the DEJ.70 However, experience over 20 years has shown that the teeth in patients with syndromic craniosynostosis are essentially normal, that is, can be restored and move under the influence of orthodontic forces, and the only issue, on occasion, is management of the child.
Review the development and eruption of the dentition and establishment of the occlusion from birth onwards
The eruption of teeth is frequently delayed in children with Apert syndrome but not to any great extent in children with other forms of syndromic craniosynostosis. We can infer from this that the mechanisms that cause teeth to erupt are the same as in non-affected individuals and the reason for the delay in Apert syndrome is currently unknown. No intervention is required if tooth eruption is delayed and it does not appear to cause any significant issues. The primary and secondary dentitions are crowded, to a variable degree, reflecting the severity of the effect of the syndrome on jaw size. The occlusion is almost always Class III with a narrow upper arch producing a bilateral posterior crossbite. The overbite is frequently reduced with anterior open bites of varying size frequently seen.71,72,73,74,75
Provide or oversee orthodontic treatment to: align the dentition, create interdental space for osteotomies and prepare for orthognathic surgery
Alignment - intervention to align the dentition is often undertaken when the degree of crowding results in teeth becoming grossly ectopic and particularly so in the maxilla. The plan is to remove teeth that cannot be aligned and, on occasion, attach gold chains to facilitate alignment using orthodontic traction. This is usually undertaken during the late mixed or early permanent dentition as an interceptive measure that is, not definitive orthodontic treatment, which is usually undertaken at a later date in combination with orthognathic surgery that is, at skeletal maturity. The introduction of cone beam CT imaging has made the 3D localisation of ectopic teeth much easier and also enables a check to be made for resorption of adjacent teeth which may influence extraction decisions.
Interdental space - the FB requires a midline maxillary osteotomy to allow the two halves of the face to be separated at the maxillary level. Space may need to be created between the upper central incisors to facilitate the osteotomy without damaging the adjacent teeth. This usually requires a short course of sectional fixed appliance treatment over a period of approximately six months.
Active management of the occlusion during major craniofacial surgery (FFA or FB) is generally not undertaken. The plan is to leave definitive occlusal correction until skeletal maturity when orthodontic preparation can be combined with orthognathic surgery. Other centres take a different approach reflecting their own treatment philosophy. There is currently no evidence to suggest that one approach is better than the other.
Finally, preparation for orthognathic surgery is no different from preparing any other patient. The basic principles of alignment, decompensation and arch coordination still apply using full upper and lower fixed appliances.76 The aim for this complex group of patients is to produce the best possible occlusion with well-aligned dental arches (Fig. 14).
Craniofacial microsomia (CFM)
This relatively common craniofacial anomaly has many synonyms: hemifacial microsomia (HFM), first and second arch syndrome and oculo-auriculo-vertebral spectrum (OAVS). HFM was first described by Gorlin as a condition with unilateral microtia, macrostomia and hypoplasia of the mandibular ramus and condyle.77 It is the most common craniofacial condition after CLP and occurs with a frequency of between 1:4,000-5,600 live births.78,79,80 It occurs bilaterally in 10-16% of cases, hence the term CFM is preferred.81,82 CFM can be associated with cardiac, neural, renal, vertebral or ocular anomalies. The male to female ratio is 3:2 and it affects the right side compared to the left similarly, 3:2.83 It is also associated with CP. Its main clinical feature is facial asymmetry, particularly of the mandible.
Aetiology of CFM
Most cases of CFM occur sporadically although autosomal dominance has been reported.84,85Poswillo86 in 1973 initiated a stapedial artery bleed in the branchial arches of an animal model that produced similar facial features of the condition. Cousley87 produced a similar phenotype in a rat model with a pre-existing mutation of chromosome 10. There are some familial cases such as discordance in monozygotic twins. Drugs such as thalidomide, retinoic acid and primidone have been implicated. Neuroectodermal death has also been implicated.
Clinical presentation and features
CFM has a broad phenotype that is, a wide spectrum of presentation. Figure 15 shows the variation of the condition. The milder cases can be relatively easy to miss. There are no agreed minimal features. Typically it presents as:
-
Mandibular asymmetry (unilateral or bilateral)
-
Orbital dystopia (vertical asymmetry of eyes)
-
Macrostomia (large mouth opening)
-
Microtia (small ear).
Other features:
-
Small skin tags or pits anterior to the tragus of the ear
-
CLP occurs in 10-25% of cases88
-
Often small for age
-
Facial nerve palsy (25-40%)
-
Airway problems, for example, OSA, anophthalmus (absent eye), middle ear defects or an absent ear (anotia)
-
Dental development can be significantly delayed
-
Increased incidence of hypodontia.
Embryology of CFM
The face develops from a series of five paired swellings of mesoderm known as the branchial arches. This occurs during the somite period at approximately four to five weeks of intrauterine life.
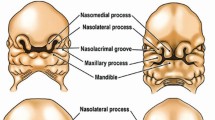
The first branchial arch is located between the stomadeum and the first pharyngeal pouch. From the maxillary and mandibular processes the bones of the lower two thirds of the face are formed. From the maxillary process the zygoma, squamous temporal, maxilla, premaxilla and palate develop. From the mandibular process, the mandible, malleus and incus of the inner ear develop. The muscles of mastication, parts of the maxillary and external carotid artery also develop. The first arch is innervated by the trigeminal nerve (Vth cranial nerve).
The second branchial arch appears later, usually 7-8 weeks, and gives rise to the stapes of the inner ear, parts of the hyoid, the stapedius muscle, part of the internal carotid artery, and the muscles of facial expression. The second branchial arch is innervated by the facial nerve (VII cranial nerve) that also supplies via chorda tympani taste to the anterior two thirds of the tongue.
At approximately the 32nd day of intrauterine life the normal process of atrophy of the stapedial artery occurs, allowing the switch of the blood supply to the face from the internal carotid to the external carotid artery (Fig. 16).
The skin tags of HFM represent epithelial remnants of the fusion of the maxillary and mandibular processes of the first branchial arch. Failure of fusion of these processes presents as macrostomia. It follows that the middle and inner ear defects of HFM are related to these anomalies of the first and second branchial arches.
Classification of CFM
The classification of the condition is best described in terms of hard and soft tissue.
Pruzansky classification
The Kaban89 modification of the Pruzansky classification is based on the bony deficiency of the mandible (Fig. 17):
Type 1 - all bony parts present but deficient or hypoplastic Type IIa - small condyle but functioning hinge joint Type IIb - joint not functioning and rudimentary condyle Type III - absence of ramus, coronoid and condyle.
Omens classification
Vento described a combined classification of soft, hard tissue and function - OMENS (orbit, mandible, ear, nerve, soft tissue).90 Each area is scored (0-3) with three being the most severe deformity score, to reach a total deformity score (Table 3). Therefore, 15 would be the maximum score possible.
Goldenhar's syndrome
There is some confusion with the number of synonyms for CFM and overlap with the descriptions of the various conditions. Goldenhar's is said to represent approximately 10% of CFM anomalies and it consists of a triad of:
-
CFM
-
Vertebral anomalies
-
Ocular anomalies – epibulbar dermoids and/or coloboma of the upper eyelid.
It is typically a unilateral condition unlike CFM. Auricular defects are seen in more than 60% of cases. Its incidence is said to be rarer, between 1:5,000-25,000 of live births. Transmission has shown autosomal dominance as well as recessive traits. The aetiology has been attributed to insecticides, drugs such as cocaine, thalidomide, tamoxiphen and maternal diabetes. The vertebral anomalies include fusion of ribs, supernumerary, hemivertebrate or agenesis of vertebrae and kyphosis. CLP may also be present.
Facial and dental features of CFM
The assessment of facial asymmetry in this group of patients is difficult to quantify. However, it has been made simpler by 3D imaging. As well as the orbital asymmetry there is often nasal deviation to the affected side. The maxilla on the affected side is generally hypoplastic, often with corresponding soft tissue deficiency. There is frequently a cant of the maxilla representing its underdevelopment on the ipsilateral side. The developing dentoalveolar process of the maxilla in the mixed dentition cannot maintain occlusal contact with the mandible. This is typically seen as a vertical deficiency in the maxillary dentition on the affected side. It is not uncommon to see a posterior open bite on the same side. The occlusion is typically on a Class II skeletal base with bimaxillary retrognathia and retrogenia, with mandibular asymmetry. The ramus and body of the mandible being hypoplastic shortens the lower arch on the affected side and crowding is usually severe in this quadrant. The lower centre line is also typically off to the affected side.
The temporomandibular joint (TMJ) may be affected depending on the severity of the condition. There is often deviation to the affected side on opening. In the more severe forms, reconstruction of the TMJ with an autologous graft is performed but may become ankylosed.
Often there is a lack of muscle bulk in the affected area and up to 25% have facial nerve (VII) weakness. This particularly affects the marginal mandibular branch of the facial nerve. This can have an effect on the symmetry of the smile, making it appear worse than the underlying skeletal deformity.
At the dental level there is an occasional association with a solitary upper central incisor.90 This is an important but rare feature as it may be an underlying sign of holoprosencephaly, a failure of division of the cerebral hemispheres or incomplete development of the brain. This confirms the neuroectodermal contribution. There may be other cardiac and skeletal features associated with CFM. Goldenhar's appears to be part of this spectrum.
Management of CFM
The management of CFM is complex and lengthy and is best undertaken in specialist units. Severe airway problems can require intubation and early surgical intervention in terms of distraction osteogenesis (DO) of the mandible to improve the mandibular position or even tracheostomy. This is rare however. Sleep studies of the more severely affected patients may show signs of obstructive sleep apnoea (OSA). The soft tissue repairs of skin tags and macrostomia are usually undertaken during the first year of life. Middle and inner ear problems are frequently seen and managed as are needed to promote speech and language development. Ear reconstruction is normally delayed until the ear is almost fully grown (7-8 years). Epibulbar dermoids can be resected or debulked but not without risk.
Orthodontic and surgical management
The very mild cases, for example, Pruzansky I may be treated in the mixed dentition with customised orthodontic hybrid appliances91 to maximise the growth potential of the maxilla and mandible of the affected side. There is some debate here as to the success of interceptive treatment with functional appliances due to the underlying soft and hard tissue deficiencies. Currently, many of the milder cases do not require any intervention in the mixed to early permanent dentition.
The more severely affected individuals, that is, Pruzansky III usually require surgical intervention with a costochondral (rib) graft or similar to encourage further mandibular growth and maximise growth potential in the maxilla. This is often undertaken at 7-10 years of age. It is well known, however, that the behaviour of costochondral growth in the mandible is unpredictable, a third of the ribs not growing, a third growing within the normal range, the remaining third presenting with overgrowth.
The Pruzansky II deformities have been traditionally treated by distraction osteogenesis of the mandible first described by McCarthy in the mixed dentition.92,93 The work of Meazzini94,95 and others has shown however that the initial aesthetic and psychological benefits can be short lived as the mandible tends to relapse towards its original position and the asymmetry reappears. As the deformity is three dimensional, it is a difficult condition to manage particularly in a growing child.
Current practice is moving away from intervention in the mixed dentition. The recent systematic review by Pluijmers96 based on 30 articles from a total of 1,611 found no statistical evidence to support the use of DO as a single treatment modality in children. The best outcomes were found in the milder cases treated with DO and grafts in the permanent dentition, usually 15 years of age. For the severely hypoplastic mandible a multistaged approach is recommended, while recognising the difficulty in establishing a single treatment protocol for such a heterogenous group of patients as CFM. Conventional bimaxillary orthognathic treatment to level any occlusal/maxillary cant and mandibular surgery with or without a genioplasty can be undertaken as required at the completion of growth. Where facial nerve deficiency warrants intervention, facial reanimation can also be considered. There is some evidence that use of temporary anchorage devices in the maxilla may reduce the need for maxillary surgery.97 Long term follow up of these cases is required to confirm initial favourable findings.
As CFM is a 3D deformity, 3D models are useful for planning such complex cases. The development of and choice of alloplastic and/or autologous grafts is increasing year on year. Soft tissue reconstruction and facial contouring has been traditionally undertaken with free soft tissue flaps. Alloplastic TMJs bulk out some of these deficiencies. Smaller defects suitable for Coleman fat transfers (autologous) are becoming more popular. Adipose tissue is taken from a donor site, thigh or abdomen (often the belly button), centrifuged intraoperatively and injected subcutaneously into the area of deficiency. The technique has evolved with improved long term benefit. It has the advantage of being minimally invasive. As a camouflage procedure it may be a reasonable alternative for an adolescent until conventional orthognathic treatment can be offered.
Finally, those patients with known mutations will need counselling from the geneticists as to their future plans.98
Treacher Collins syndrome
Treacher Collins syndrome (TCS) is also known as mandibulofacial dysostosis. This largely autosomal dominant condition has a penetrance of approximately 90% and variable expressivity.99 Its incidence is much rarer, affecting 1:50,000 of live births. Its features are bilateral, generally symmetrical and include (Fig. 18):
-
Microtia 77% or anotia
-
Conductive deafness 50%
-
Zygoma deficiency 80%
-
Mandibular hypoplasia 78%
-
Downward slanting palpebral fissures
-
Lower eyelid coloboma 75%
-
Cleft palate 30% plus facial clefting is seen in some
-
Absence of lower medial third of eyelashes.
Developmental delay is rare in TCS. Occasionally there is severe respiratory distress in neonates with micrognathia. Intervention with an airway or distraction of the mandible may be indicated in preference to a tracheostomy.
Aetiology and genetics
This is a first and second arch branchial arch defect. Types 1&2 have autosomal dominance inheritance, type 3 has autosomal recessive trait.
Gene TCOF1 (Treacher Collins syndrome 1) OMIM 154,500
Gene POLR1D (Treacher Collins syndrome 2) OMIM 613,717
Gene POLR1C (Treacher Collins syndrome 3) OMIM 248,390
The genetic defect is unknown for about 10% of TC diagnosis. In most cases the mutation is in the gene TCOF1 located on 5q32-q33.1 chromosome. This gene encodes the treacle phosphoprotein essential for normal cell growth.
Facial and dental features
There is frequently a significant underlying Class II skeletal discrepancy with significant shortening of the rami of the mandible, antegonal notching of the mandible and a clockwise rotation of the occlusal plane with an increased MM angle. TCS often has a Tessier facial cleft 6-8. As a result of the hypoplastic skeletal features, there is typically severe crowding of the dentition.
Conclusion
The aesthetic, functional and psychological management of patients with craniofacial malformations presents a great challenge to the members of the MDT. The timing and sequence of interventions needs to be carefully coordinated with all members of the team and in particular the psychologists and nurse specialists. Close liaison with primary and secondary care – both medical and dental – is essential to ensure that all aspects of care are provided.
The diagnosis and treatment for the rarer conditions is challenging due to the variation in phenotype and presentation. A recent editorial in the Journal of Orthodontics suggested the treatment of HFM and cleidocranial dysostosis should be centralised, as it is for CLP and syndromic craniosynostosis.57 This could be one way to build on the experience and skill of the multidisciplinary teams looking after these patients.
References
Fraser F C . The genetics of cleft lip and cleft palate. Am J Hum Genet 1970; 22: 336–352.
Gorlin R J, Cohen M M Jr, Hennekam R C M . Syndromes of the head and neck. New York: Oxford University Press, 2001.
Bellis T H, Wohlgemuth B . The incidence of cleft lip and palate deformities in the south-east of Scotland. Br J Orthod 1999; 26: 121–125.
Schutte B C, Murray J C . The many faces and factors of orofacial clefts. Hum Mol Genet 1999; 8: 1853–1859.
Mitchell L E, Risch N . Mode of inheritance of non syndromic cleft lip with or without cleft palate: a reanalysis. Am J Hum Genet 1992; 51: 323–332.
Cobourne M T . The complex genetics of cleft lip and palate. Eur J Orthod 2004; 26: 7–15.
Kondo S, Schutte B C, Richardson R J et al. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet 2002; 32: 285–289.
Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms J A, Spritz R A . Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpes virus receptor, in cleft lip/palate ectodermal dysplasia. Nat Genet 2000; 25: 427–430.
Mossey P A, Little J, Munger R G, Dixon M J, Shaw W C . Cleft lip and palate. Lancet 2009; 374: 1773–1783.
Little J, Gilmour M, Mossey P A et al. Folate and clefts of the lip and palate: a UK based case-control study, part 1dietary and supplemental folate. Cleft Palate Craniofac J 2008; 45: 420–427.
Meyer K A, Werler M M, Hayes C, Mitchell A A . Low maternal alcohol consumption during pregnancy and oral clefts in offspring: the Sloane Birth Defects Study. Birth Defects Res A Clin Mol Teratol 2003; 67: 509–514.
Botto L D, Erickson J D, Mulinare J, Lynberg M C, Liu Y . Maternal fever, multivitamin use, and selected birth defects: evidence of interaction? Epidemiology 2002; 13: 485–488.
Johnson C Y, Little J . Folate intake, markers of folate status and oral clefts: is the evidence converging? Int J Epidemiol 2008; 37: 1041–1058.
Kriens O . LAHSHAL a concise documentation system for cleft lip, alveolus and palate diagnosis. In: What is cleft lip and palate? New York: Thieme Publishing Group, 1989.
Royal College of Surgeons England, 1995.
Shaw W C, Dahl E, Asher-McDade C et al. A six-center international study of treatment outcome in patients with clefts of the lip and palate: Part 5. General discussion and conclusions. Cleft Palate Craniofac J 1992; 29: 413–418.
Mars M, Plint D A, Houston W J, Bergland O, Semb G . The Goslon Yardstick: a new system of assessing dental arch relationships in children with unilateral clefts of the lip and palate. Cleft Palate J 1987; 24: 314–322.
Mars M, Asher-McDade C, Brattström V et al. A six-center international study of treatment outcome in patients with clefts of the lip and palate: Part 3. Dental arch relationships. Cleft Palate Craniofac J 1992; 29: 405–408.
Bearn D, Mildinhall S, Murphy T et al. Cleft lip and palate care in the United Kingdom - the Clinical Standards Advisory Group (CSAG) Study. Part 4: outcome comparisons, training, and conclusions. Cleft Palate Craniofac J 2001; 38: 38–43.
Sandy J, Williams A, Mildinhall S et al. The Clinical Standards Advisory Group (CSAG) Cleft Lip and Palate Study. Br J Orthod 1998; 25: 21–30.
Clinical Standards Advisory Group (CSAG). Cleft lip and/or palate. Report of a CSAG Committee. London: The Stationery Office, 1998.
Young J L, O'Riordan M, Goldstein J A, Robin N H . What information do parents of newborns with cleft lip, palate, or both want to know? Cleft Palate Craniofac J 2001; 38: 55–88.
Wolf L S, Glass R P . Feeding and swallowing disorders in infancy, assessment and management. Arizona Therapy Skill Builders, 1999.
Grunwell P, Sell D A . Management of cleft lip and palate. London: Whurr Publishing, 2001.
Spreistersbach D C, Dickson D R, Fraser F C et al. Clinical research in cleft lip and palate: the state of the art. Cleft Palate J 1973; 10: 113–165.
Sheahan P, Miller I, Sheahan J N, Earley M J, Blayney A W . Incidence and outcome of middle ear disease in cleft lip and/or cleft palate. Int J Pediatr Otorhinolaryngol 2003; 67: 785–793.
Gaggl A, Schultes G, Kärcher H . Aesthetic and functional outcome of surgical and orthodontic correction of bilateral clefts of lip, palate and alveolus. Cleft Palate Craniofac J 1999; 36: 407–412.
Ranta R . A review of tooth formation in children with cleft lip/palate. Am J Orthod Dentofacial Orthop 1986; 90: 11–18.
Harris E F, Hullings J G . Delayed dental development in children with isolated cleft lip and palate. Arch Oral Biol 1990; 35: 469–473.
Olin W H . Dental anomalies in cleft lip and cleft palate patients. Angle Orthod 1964; 34: 119–123.
Dewinter G, Quirynen M, Heidbüchel K, Verdonck A, Willems G, Carels C . Dental abnormalities, bone graft quality, and periodontal conditions in patients with unilateral cleft lip and palate at different phases of orthodontic treatment. Cleft Palate Craniofac J 2003; 40: 343–350.
Van den Boogaard M J, Dorland M, Beemer F A, van Amstel H K . MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet 2000; 24: 342–343.
Bonh A . The course of the premaxillary and maxillary vessels and nerves in cleft jaw. Acta Odontol Scand 1963; 21: 463–513.
Bondemark L, Tsiopa J . Prevalence of ectopic eruption, impaction, retention and agenesis of the permanent second molar. Angle Orthod 2007; 77: 773–778.
Fox N A, Fletcher G A, Homer K . Localising maxillary canines using dental panoramic tomography. Br Dent J 1995; 179: 416–420.
Bjerklin K, Kurol J, Paulin G . Ectopic eruption of the maxillary first permanent molars in children with cleft lip and/or palate. Eur J Orthod 1993; 15: 535–540.
Akcam M O, Toygar T U, Ozer L, Ozdemir B . Evaluation of 3-dimensional tooth crown size in cleft lip and palate patients. Am J Orthod Dentofacial Orthop 2008; 134: 85–92.
Dixon D A . Defects of structure and formation of the teeth in persons with cleft palate and the effect of reparative surgery on the dental tissues. Oral Surg Oral Med Oral Pathol 1968; 25: 435–446.
Lucas V S, Gupta R, Ololade O, Gelbier M, Roberts G J . Dental health indices and caries associated microflora in children with unilateral cleft lip and palate. Cleft Palate Craniofac J 2000; 37: 447–452.
Dahllöf G, Ussisso-Joandi R, Ideberg M, Modeer T . Caries, gingivitis and dental abnormalities in preschool children with cleft lip and/or palate. Cleft Palate J 1989; 26: 233–238.
Wong F W, King N M . The oral health of children with clefts - a review. Cleft Palate Craniofac J 1998; 35: 248–254.
Hasslöf P, Twetman S . Caries prevalence in children with cleft lip and palate - a systematic review of case-control studies. Int J Paediatr Dent 2007; 17: 313–319.
Hermann N V, Jensen B L, Dahl E, Bolund S, Darvann T A, Kreiborg S . Craniofacial growth in subjects with unilateral complete cleft lip and palate, and unilateral incomplete cleft lip, from 2 to 22 months of age. J Craniofac Genet Dev Biol 1999; 19: 135–147.
Hayashi I, Sakuda M, Takimoto K, Miyazaki T . Craniofacial growth in complete unilateral cleft lip and palate: a roentgeno-cephalometric study. Cleft Palate J 1976; 13: 215–237.
Abd Rahman N, Abdullah N, Samsudin A R, Naing Mohd Ayub Sadiq L . Dental anomalies and facial profile abnormality of the non-syndromic cleft lip and palate children in kelantan. Malays J Med Sci 2004; 11: 41–51.
Mars M, Houston W J B . A preliminary study of facial growth and morphology in unoperated male unilateral cleft lip and palate subjects over 13 years of age. Cleft Palate J 1990; 27: 23–31.
Ireland A J, McDonald F . The orthodontic patient: treatment and biomechanics. Oxford University Press, 2003.
Mendelian inheritance in man. https://www.ncbi.nlm.nih.gov/omim
Buchanan E P, Xue A S, Hollier L H Jr . Craniofacial syndromes. Plast Reconstr Surg 2014; 134: 128e–153e.
Johnson D, Wilkie A O M . Craniosynostosis. Eur J Hum Genet 2011; 19: 369–376.
Hayward R, Jones B, Dunaway D, Evans R (eds). The clinical management of craniosynostosis. Clinics in Developmental Medicine No. 163. London: Mac Keith Press, 2004.
Evans R D . Highly specialised services. J Orthod 2013: 40: 181–182.
Meikle M C . Reconstructing faces. The art and wartime surgery of Gillies, Pickerill, McIndoe and Mowlem. Otago University Press, 2013.
Ortiz-Monasterio F, del Campo A F, Carrillo A . Advancement of the orbits and midface in one piece combined with forehead repositioning, for the correction of Crouzon's deformity. Plast Reconstr Surg 1978; 61: 507–516.
Van der Meulen J C . Median faciotomy. Br J Plast Surg 1979; 32: 339–342.
Tessier P . Apert syndrome: Acrocephalosyndactyly type 1. In Carronni E P (ed). Craniofacial surgery. pp 280–303. Boston: Little Brown, 1985.
Ilizarov G A . Basic principles of transosseous compression anddistraction osteosynthesis. Ortop Travmatol Protez 1971; 32: 7–15.
Ilizarov G A . The principles of the Ilizarov method. 1988. Bull Hosp Jt Dis 1997; 56: 49–53.
McCarthy J G, Schreiber J, Karp N, Thorne C H, Grayson B H . Lengthening the human mandible by gradual distraction. Plast Reconstr Surg 1992; 89: 1–8.
Adolphs N, Ernst N, Menneking H, Hoffmeister B . Significance of distraction osteogenesis of the craniofacial skeleton – a clinical review after 10 years of experience with the technique. J Craniomaxillofac Surg 2014; 42: 966–975.
Heggie A A, Kumar R, Shand J M . The role of distraction osteogenesis in the management of craniofacial syndromes. Ann Maxillofac Surg 2013; 3: 4–10.
Greig A V H, Britto J A, Abela C et al. Correcting the typical Apert face: combining bipartition with distraction. Plastr Reconstr Surg 2013; 131: 219e–230e.
Dunaway D J, Brittto J B, Abela C, Evans R D, Jeelani N U . Complications of frontofacial advance. Childs Nerv Syst 2012; 28: 1571–1576.
Sant'Anna E F, de A Cury-Saramago A, Figueroa A A, Polley J W . Evaluation of maxillary permanent molars in patients with syndromic craniosynostosis after monobloc osteotomy and midface advancement with rigid external distraction (RED). Cleft Palate Craniofac J 2010; 47: 109–115.
Vagervik K, Rubin M S, Grayson B H et al. Parameters of care for craniosynostosis: dental and orthodontic perspectives. Am J Orthod Dentofacial Orthop 2012; 141: S68–S73.
Mustafa D, Lucas V, Junod P, Evans R, Mason C, Roberts G . The dental health and caries related microflora in children with craniosynostosis. Cleft Palate Craniofac J 2001; 38: 629–635.
Dalben Gda, Cosat B, Gomide M R . Oral health status of children with syndromic craniosynostosis. Oral Health Prev Dent 2006; 4: 173–179.
Múfalo P S, Kaizer Rde O, Dalben Gda S, de Almeida A L . Comparison of periodontal parameters in individuals with syndromic craniosynostosis. J Appl Oral Sci 2009; 17: 13–20.
Geels L M, Kieffer J M, Hoogstrayen J, Prahl-Anderson B . Oral health-related quality of life of children with craniofacial conditions. Cleft Palate Craniofac J 2008; 45: 461–467.
Surman T L, Logan R M, Townsend G C, Anderson P J . Oral features in Apert syndrome: a histological investigation. Orthod Craniofac Res 2010; 13: 61–67.
Kreiborg S, Cohen M M Jr . The oral manifestations of Apert syndrome. J Craniofac Genet Dev Biol 1992; 12: 41–48.
Kaloust S, Ishii K, Vagervik K . Dental development in Apert syndrome. Cleft Palate Craniofac J 1997; 34: 117–121.
Reitsma J H, Balk-Leurs I H, Ongkosuwito E M, Wattel E, Prahl-Andersen B . Dental maturation in children with the syndrome of crouzon and apert. Cleft Palate Craniofac J 2013; 51: 639–644.
Reitsma J H, Ongkosuwito E M, Buschang P H, Prahl-Andersen B . Facial growth in patients with apert and crouzon syndromes compared to normal children. Cleft Palate Craniofac J 2012; 49: 185–193.
Reitsma J H, Elmi P, Ongkosuwito E M, Buschang P H, Prahl-Andersen B . A longitudinal study of dental arch morphology in children with the syndrome of Crouzon or Apert. Eur J Oral Sci 2013; 121: 319–327.
Ayoub A, Khambay B, Benington P, Green L, Moos K, Walker F (eds). (2013). Basic orthognathic surgical procedures. In: Handbook of orthognathic treatment: a team approach. Oxford, UK: John Wiley & Sons, Ltd 2013.
Gorlin R J, Pindborg J J, Cohen M M Jr . Syndromes of the head and neck, 2nd ed. pp 261–265. New York: McGraw-Hill, 1976.
Converse J M, Coccaro P J, Becker M, Wood-Smith D . On hemifacial microsomia. The first and second branchial arch syndrome. Plast Reconstr Surg 1973; 51: 268–279.
Grabb W C . The first and second branchial arch syndrome. Plast Reconstr Surg 1965; 36: 485–508.
Kelberman D, Tyson J, Chandler D C et al. Hemifacial microsomia: progress in understanding the genetic basis of a complex malformation syndrome. Hum Genet 2001; 109: 638–645.
Grayson B H, Boral S, Eisig S, Kolber A, McCarthy J G . Unilateral craniofacial microsomia. Part 1. Mandibular analysis. Am J Orthod 1983; 84: 225–230.
Horgan J E, Padwa B L, LaBrie R A, Mulliken J B . OMENS-Plus: analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft Palate Craniofac J 1995; 32: 405–412.
Rollnick B R, Kaye C I, Nagatoshi K, Hauck W, Martin A O . Oculoauriculovertebral dysplasia and variants: phenotypic characteristics of 294 patients. Am J Med Genet 1987; 26: 361–375.
Strömland K, Miller M, Sjögreen L et al. Oculo-auriculo-vertebral spectrum: associated anomalies, functional deficits and possible developmental risk factors. Am J Med Genet A 2007; 143A: 1317–1325.
Vendramini-Pittoli S, Kokitsu-Nakata N M . Oculoauriculovertebral spectrum: report of nine familial cases with evidence of autosomal dominant inheritance and review of the literature. Clin Dysmorphol 2009; 18: 67–77.
Poswillo D . The pathogenesis of the first and second branchial arch syndrome. Oral Surg Oral Med Oral Pathol 1973; 35: 302–328.
Cousley R, Naora H, Yokoyama M, Kimura M, Otani H . Validity of the Hfm transgenic mouse as a model for hemifacial microsomia. Cleft Palate Craniofac J 2002; 39: 81–92.
Pruzansky S . Not all dwarfed mandibles are alike. Birth Defects 1969; 5: 120–129.
Kaban L B, Moses M H, Mulliken J B . Surgical correction of hemifacial microsomia in the growing child. Plast Reconstr Surg 1988; 82: 9–19.
DiBiase A T, Cobourne M T . Beware the solitary maxillary median central incisor. J Orthod 2008; 35: 16–19.
Vig P S, Vig K W . Hybrid appliances; a component approach to dentofacial orthopedics. Am J Orthod Dentofacial Orthop 1986; 90: 273–285.
McCarthy J G, Schreiber J, Karp N, Thorne C H, Grayson B H . Lengthening the human mandible by gradual distraction. Plast Reconstr Surg 1992; 89: 1–8.
Kaban L B, Padwa, B L, Mulliken J B . Surgical correction of mandibular hypoplasia in hemifacial microsomia; the case for treatment in early childhood. J Oral Maxillofac Surg 1998 56: 628–638.
Meazzini M C, Mazzoleni F, Canzi G, Bozzetti R Mandibular distraction osteogenesis in hemifacial microsomia: long-term follow-up. J Craniomaxillofac Surg 2005; 33: 370–376.
Meazzini M C, Mazzoleni F, Bozzetti R, Brusati R, Does functional appliance treatment truly improve stability of mandibular vertical distraction osteogenesis in hemifacial microsomia? J Craniomaxillofac Surg 2008; 36: 384–389.
Pluijmers B I, Caron C J, Dunaway D J, Wolvius E B, Koudstaal M J . Mandibular reconstruction in the growing patient with unilateral craniofacial microsomia: a systematic review. Int J Oral Maxillofac Surg 2014; 43: 286–295.
Cousley R R, Turner M J . Mini-implant applications in orthognathic surgical treatment. J Orthod 2014; 41: s54–s61.
Wilkie A O, Bochukova E G, Hansen R M et al. Clinical dividends from the molecular genetic diagnosis of craniosynostosis. Am J Med Genet A 2006; 140: 2631–2639.
Dixon J, Trainor P, Dixon M J . Treacher Collins syndrome. Orthod Craniofac Res 2007; 10: 88–95.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Akram, A., McKnight, M., Bellardie, H. et al. Craniofacial malformations and the orthodontist. Br Dent J 218, 129–141 (2015). https://doi.org/10.1038/sj.bdj.2015.48
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2015.48