Key Points
-
Outlines the prevalence of halitosis in the general population, its classification and psychosocial implications.
-
Reports that about 10% of genuine halitosis cases are caused by extra-oral phenomena, giving extra-oral halitosis an estimated prevalence of about 0.5-3% in the general population.
-
Reviews the causes of extra-oral halitosis and how to differentiate these from intra-oral halitosis.
Abstract
Halitosis is a symptom and not a diagnosis. Rather, the topic represents a spectrum of disorders, including intra-oral, otorhinolaryngological, metabolic, systemic, pulmonary, psychological and neurological conditions. Halitosis may be the third most common trigger for patients to seek dental care and can cause significant impact on patient quality of life. About 10% of all genuine halitosis cases are attributed to extra-oral processes. Some authorities have reported that the nasal cavity and the oropharynx are the most common sites of origin of extra-oral halitosis. However, recent evidence appears to suggest that blood borne halitosis may be the most common subtype of extra-oral halitosis. Tangerman and Winkel report that dimethyl sulphide was the main volatile implicated in extra-oral blood borne halitosis. They proposed a hitherto unknown metabolic condition by way of explanation for this finding, resulting in systemic presence of dimethyl sulphide in blood and alveolar breath. This paper reviews the knowledge base regarding the behaviour of dimethyl sulphide in physiological systems and those disorders in which blood borne halitosis secondary to dimethylsulphidemia is thought to have an aetiopathological role.
Similar content being viewed by others
Background
Several definitions of the term halitosis have been offered. It is generally accepted that odours on the nose breath, as well as the mouth breath, are included within this topic. Similarly included are those conditions that present with more pleasant breath odours eg, the 'fruity' breath of diabetic ketoacidosis and also those cases where there is no genuine odour in a patient complaining of halitosis, a scenario which has a myriad of causes.
Some have called for standardisation of protocols in halitosis research.1 Perhaps due to a lack of consensus regarding diagnostic criteria and assessment methods, the reported prevalence varies, but this value is probably about 20%.2,3,4,5
As such, it is rightly described as a common symptom6 and may even be the third most common trigger to seek dental care, (after dental caries and periodontal diseases),7,8 showing that patients place great importance on it.
Halitosis is subject to great societal taboo and stigma.6,9 The evolutionary function of olfaction in humans serves to detect spoiled food sources and potentially infectious or harmful stimuli.10,11 It also has a role in mate selection.12,13,14,15,16 Unpleasant personal odour triggers repulsion in others. This is thought to be an evolutionary response to avoid mating with sick individuals.17,18 The need to belong has been identified as a fundamental human need.19 Social rejection and ostracism has been shown to have profound physical and psychological effects.20,21 This symptom can therefore have significant psychosocial implications, including social anxiety and avoidance behaviours, problems with integration, relationships and productivity, depression and reduced quality of life.6,22,23,24,25 It has been suggested that clinicians are poorly informed about the causes and treatments for halitosis, and may even mismanage cases, promoting adverse psychological sequalae.8,26 It is also suggested that clinicians may need to be updated regarding the current evidence base.27
Non-genuine halitosis
A proportion of patients who present complaining of halitosis will not have any detectable breath malodour. Various terms have been used to describe this, including delusional halitosis, psychogenic halitosis, pseudohalitosis and halitophobia. Estimates of non-genuine halitosis among those complaining of halitosis range from 5-72%.6,28,29,30,31,32,33,34 There are several possible reasons why a finding of non-genuine halitosis could be made.
Assessment errors and symptom transience
Malodour may have been present at the time of consultation and was not detected. Those involved in the management of patients complaining of halitosis ideally would be familiar with the principles of organoleptic assessment35,36 or even have access to breath analysis apparatus (for example, the 'OralChroma™').37 It is also possible that malodour was not present at the time of the assessment but may be present at other times. Clinicians frequently fail to recognise the variability of the presence and strength of the symptom.38 Factors such as hydration, timing and nature of last food consumption, time of day, menstrual status, nature of clinical environment and fluctuations in clinician olfactory acuity possibly affecting the detection threshold on any single occasion.
Genuine bad tastes and dysgeusia
Although gustation and olfaction are closely related senses, a bad taste often does not necessarily translate to detectable malodour by others. Disorders of taste can present as a complaint related to smell and vice versa. Genuine bad tastes can be caused by any number of factors, including poor oral hygiene, dental caries, periodontal diseases (for example, acute necrotising ulcerative gingivitis), oral cancer, diabetes and uraemia.39
Patients may also experience dysgeusia (taste disturbance), which has many causes, commonly smoking, xerostomia, ageing, infection, trauma involving the mouth, nose or head, or nerve damage involving the chorda tympani, lingual or facial nerves, gastro-oesophageal reflux disease (GORD) and deficiency states (B12 or zinc). For a more extensive list of the rarer causes of dysgeusia, readers are directed to dedicated reviews of the topic.40,41,42,43 Iatrogenic effect of various medications may cause both genuine bad tastes and dysgeusia.
Dysosmia (olfactory disturbance)
Parosmia is the misinterpretation or distortion of odour stimuli and may occur with upper respiratory tract infections, head trauma or accompany ageing.44
Phantosmia is perception of a smell in the absence of any odour stimuli. Phantosmia can occur with conditions such as alcoholic psychosis, pregnancy, chemo- or radiotherapy, Parkinson's disease, Alzheimer's disease, schizophrenia, temporal lobe epilepsy or lesions impinging upon the olfactory bulb or tract (for example, a meningioma or neuroblastoma).45,46,47,48
Patients with genuine halitosis develop olfactory fatigue when their symptom is present, making them poor judges of their own odour.49 Often, the only trigger for patients to medical or dental advice is the behaviour of those they come into contact with. Conversely, patients with phantosmia may report self detection of an odour which others do not seem to comment on.
Psychological condition
Halitophobia can be defined as follows, 'when there is no physical or social confirmation to suggest that halitosis is present, which can persist after therapy for either genuine halitosis or pseudohalitosis'.35 Some consider halitophobia to be a type of olfactory reference syndrome (ORS), where there is preoccupation with the belief that one emits a foul or offensive odour, although this odour is not perceived by others.50 These patients are said to misinterpret the behaviour of others, reinforcing this preoccupation and engage in obsessive activities such as excessive bathing or oral hygiene. ORS is not, however, included as a diagnosis in DSM-IV, and will only be included under 'conditions that require further research' in DSM-5. The persistence of adverse psychosocial sequalae after treatment for genuine halitosis is perhaps testament to the distress it can cause in some patients. Distressed patients may display behaviours that inappropriately prompt clinicians to assume a tendency towards delusional symptoms.
Recently, Falcão et al. challenged the current concepts of pseudohalitosis and halitophobia, drawing attention to altered chemo-sensory function states, but also proposed that retronasal olfaction may offer explanation for halitosis complaints that cannot be detected by others.51
The author therefore advises that a finding of non-genuine halitosis not be made without a full and competent halitological consultation, or be based upon the findings of a single occasion. Furthermore, this finding should not serve as a diagnosis, as it is a symptom in its own right with many possible causes that may warrant further investigation and management.
Genuine halitosis
Where the malodour is real and detectable, this is known as genuine halitosis. Estimations of the proportion of genuine halitosis cases among those complaining of halitosis range from 28-95%.6,28,29,30,31,32,33,34,52 Genuine halitosis is then sub-classified according to the location of the origin of the malodour, namely, intra- or extra-oral halitosis.
Intra-oral halitosis (oral malodour)
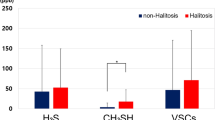
About 90% of genuine halitosis cases have a cause within the oral cavity, that is, intra-oral halitosis (oral malodour).6,28,29,30,31,32,33,34 The leading cause of intra-oral halitosis is release of volatile sulphur compounds (VSC) from halitogenic biofilm on the posterior dorsal tongue, and/or within gingival crevices/periodontal pockets. The causative organisms are usually gram-negative, anaerobic spp. VSC are generated by bacterial degradation of sulphur containing amino acid substrates, for example, methionine, cystine and cysteine.35 The main odourants implicated in intra-oral halitosis are methyl mercaptan (MM, CH3SH) and hydrogen sulphide (H2S).53 Intra-oral halitosis has been the subject of increasingly detailed study in recent times and the symptom could be considered clinically manageable in the vast majority of cases, provided a well informed clinician and motivated patient. Readers are directed to the many comprehensive reviews available on the subject.6,35,36,54,55,56,57,58,59,60,61,62,63,64,65
Extra-oral halitosis
Some 10% of genuine halitosis cases are not caused by intra-oral phenomena, giving extra-oral halitosis an estimated prevalence of 0.5-3% in the general population.28,30,32 Extra-oral halitosis is by contrast less well researched and understood and represents a greater diagnostic and therapeutic challenge. It has been classified according to location and aetiology (Table 1).27,35,36,37,66
Traditionally, it was thought that the stomach was responsible for many cases of halitosis, however, it has now been shown that only vary rarely is this the case. The oesophagus is a collapsed tube (except during deglutition, eructation, or emesis), denying the transit of odourant volatiles through to the aerodigestive tract.66,67 Gastrointestinal conditions, for example, Helicobacter pylori infection and GORD, have been investigation with regards a potential relationship with halitosis.68,69,70,71,72,73,74,75,76,77,78,79,80,81,82 Currently, the overall picture appears to be a lack of positive correlation or unconvincing evidence.
Otorhinolaryngolocial pathoses are thought by many researchers to account for the majority of extra-oral causes of halitosis, especially tonsillar29 and sino-nasal conditions.83 Others suggest that obstructive nasal pathology can secondarily cause mouth breathing, with resultant xerostomia and halitosis.84 It is known that mouth-breathing is associated with halitosis.85 With regards the proportion of genuine halitosis cases that secondary to ear nose and throat (ENT) conditions, reported estimates range from between 2.9-10%.6,30,34,86,87
However, recent evidence suggests that a mechanism known as blood borne halitosis may account for the majority of extra-oral halitosis.32
Blood borne halitosis
It has long been known that volatile chemicals present in the blood can be transferred to the exhaled breath. Breath analysis for trace chemicals offers a non invasive investigation that can be applied in a huge range of conditions. For example, hydrogen and/or methane breath analysis can be carried out following oral administration of poorly absorbable carbohydrate (for example, lactulose).88 Hydrogen and methane are products of gut microbiota fermentation, hence this breath test is thought to be a measure of gut microbiological activity. This testing is carried out by some in conditions like small intestinal bacterial overgrowth (SIBO),89 although somewhat controversial due to imperfect sensitivity. These odourless gases have been absorbed from the gut and transferred to the alveolar breath.90 The same VSC that are reported to be the most important volatiles in halitosis (H2S, MM and DMS) are implicated as being the greatest contributors to the malodorous character of flatus and faeces.91,92,93 Of this trio, only DMS is stable in blood and capable of creating blood borne halitosis.
The general mechanism involved in blood borne halitosis is as follows. Volatiles enter the systemic circulation (several routes possible but most usually via the portal system following absorption from the gut), and are then circulated to the lungs, where there is intimate associated between the pulmonary alveoli and capillary networks. During gas exchange of waste carbon dioxide and inhaled oxygen, chemicals present in the blood, may also be exchanged, and may be perceptible on the exhaled breath if they fulfil certain criteria (Table 2). The detection of a volatile on the breath by objective measurement does not imply its contribution to perceptible malodour. Although all odourants are volatile, not all volatiles are odourants. An odourant must also be present in high enough concentrations to stimulate olfactory detection. For this to happen, great enough quantities of the volatile must be liberated from solution (that is, respiratory tract secretions, saliva). Whether the chemical is liberated into the gas phase, and in what quantities, is dependent upon such factors as pH and temperature of the solution and the concentration of the volatile in the solution.
However, since the blood goes almost everywhere, blood borne volatiles may also be expressed via other routes of excretion, for example, sweat, saliva and urine. An example of a blood borne volatile being expressed via multiple routes, and not just the breath, is trimethylamine (TMA),94 which has a fish-like odour in vitro, becoming ammoniacal at higher concentrations. Indeed when TMA is present in the urine, the rare disorder trimethylaminuria (TMAU) should be considered, although there are other causes of elevated urine TMA.95 Dimethylglycinuria is another rare cause of fish odour.96
Tangerman and Winkel32 reported that dimethyl sulphide (DMS, CH3SCH3) was the most common volatile in extra-oral halitosis, which could not be attributed to recent ingestion of volatile foodstuffs. They estimated this DMS-related blood borne halitosis may affect some 0.25-1.5% of the general population. These estimates were based upon a cohort of 58 patients complaining of halitosis, and the findings extrapolated assuming 10-30% prevalence of halitosis in the general population and 5-10% of genuine halitosis being extra-oral in aetiology.32
Multidisciplinary management
The classification into intra- and extra-oral, and the sub-classification of extra-oral halitosis is useful as it guides clinical practice according to which disciplines may need to be involved,97 eg, ENT for upper respiratory tract and respiratory medicine for lower respiratory tract. The referral in blood borne halitosis is cause-related, for example, endocrinology.
There are in existence a handful of joint halitosis clinics, sometimes consisting of specialists in ENT, periodontology and psychiatry. Some suggest that this problem is best managed at such multidisciplinary centres. Practically, however, general dentistry is the primary setting involved, given that most patients will first present to the dentist and that the majority of causes of halitosis are intra-oral.
The role of the dentist in management of halitosis is to take a history and carry out a meticulous intra-oral and oropharyngeal examination for halitogenic factors. This is followed by an organoleptic assessment, to ascertain the presence or absence of malodour. Absence of any detectable malodour at repeated consultations likely represents non-genuine halitosis. It is inappropriate for these patients to receive treatments aimed at odour reduction. Simple reassurance may be carried out in general practice, however, as discussed above, non-genuine halitosis complaints may represent psychiatric or neurological conditions and referral may be indicated. Malodour that is more detectable on the mouth breath compared to the nose breath may indicate intra-oral halitosis, whereas the reverse (ie nasal foetor) may indicate sino-nasal pathology. Malodour that is equally objectionable on both the nose and the mouth breath usually indicates extra-oral halitosis.
The dentist may then treat any clinical intra-oral halitosis, or to refer if extra-oral halitosis is suspected. Further investigation of extra-oral halitosis it is perhaps more appropriately carried out by relevant specialities.98 Equally, dentists may need to be aware of patients who are referred from general medical practitioners and who are found to have signs of extra-oral halitosis.
Dimethyl sulphide, in vivo behaviour: synthesis, metabolism and excretion
DMS is a volatile organosulphur compound, constituting a sulphur atom covalently bonded to two methyl groups. The in vitro odour character has been variably described as wild radish or cabbage-like,99 or unpleasantly sweet,32 but invariably the terms used have unpleasant connotations. DMS was shown to reduce mood compared to more pleasant scents.100 DMS has an exceptionally low odour detection threshold, meaning that it is detectably malodorous even at very low concentrations (24-100 ppb).53 The gas can be fatally noxious101 and it is also a mild skin and eye irritant,102 although even at the pathological concentrations reported in disease this is probably not a feature.
In mammals, free DMS is the only form known to be present.91 DMS can be catabolised from dietary precursors in plants (S-methylmethionine/vitamin U, and dimethylsufoniopropionate).91,103,104 DMS can be synthesised from transamination of methionine,105 or methylation of methyl mercaptan (which itself can be methylated from H2S).106,107 Methylation of H2S and MM is thought to be a detoxification pathway.91 Dimethylsulphoxide (DMSO) can be reduced to DMS.102,108 Bacterial spp. known to synthesise DMS have been demonstrated in the human GI tract.109,110,111 DMS is a component of flatus and faeces91,112 and it is assumed that this is the result of intestinal bacterial activity. There is evidence that suggests of all the VSC produced in the colon >90% are absorbed by the lining rather than being emitted as flatus, and H2S and MM are thought to be metabolised by caecal lining tissue (to thiosulphate). DMS, however, is not broken down here, instead passing straight to blood.113,114,115 Free DMS is neutral molecule, it does not contain a reactive thiol group (unlike MM and H2S), hence it is stable in blood as it is unreactive with proteins. MM and H2S both react rapidly in blood and are therefore not implicated in blood borne halitosis.32 DMS that is absorbed into the bloodstream appears to be a substrate of both CYP450 and flavin containing mono-oxygenases (FMO).116,117
DMS is known to be subject to renal and pulmonary excretion.102,118,119 Dietary loading with either methionine or DMS also produced DMS in the milk of cows.120 After the work of Tangerman's group, where Tenax trapping and gas chromatography was used to objectively measure free DMS concentrations, the normal range can be taken as <7 nM in peripheral venous blood.121 Breath DMS is also very low in health. Normal subjects in one study were reported to have a breath DMS range of 0.13-0.65 nM.122 Urine DMS is reported as being generally below the 3 nM detection minimum for gas chromatography.91
Conditions where elevated blood DMS is a feature
The author suggests the term dimethylsulphidemia (DMSE, or hyperdimethylsulphidemia) to describe abnormal elevations of DMS in the blood. DMSE could be defined as >7 nM (normal range <7 nM). The threshold above which blood DMS begins to cause blood borne halitosis is undefined, although there was close correlation between blood and breath DMS reported.91,121 DMSE, (with corresponding elevated breath DMS and 'dimethylsulphiduria'), is a feature of several dif erent conditions (Table 3), and it therefore should be considered a sign rather than a diagnosis.
Apart from the conditions listed in Table 3, several curious correlations of breath DMS have been reported. Old age, female gender, HDL cholesterol, history of colon polyps and asthma were all shown to correlate with breath DMS above the organoleptic threshold.123 Elevated MM on the mouth air also correlated with DMS, possibly since MM is methylated to DMS. When investigating breath DMS attributed to DMSE, some researchers work around this artefact by measuring DMS on both mouth breath and nose breath (the small amount of DMS that could be attributed to methylation of MM intra-orally would not be detectable on the nose breath). Male pattern baldness was also correlated to elevated breath DMS in elderly men and this relationship was stronger in the presence of GI tract and metabolic disorders.124 Interestingly, cystic fibrosis patients demonstrated a lower breath DMS than a healthy control arm.125 Perhaps this could be attributed to altered gas exchange efficiency and pathological changes in pulmonary and gastrointestinal secretions. Occupational exposure in certain industries can lead to elevated exposure to DMS, which is demonstrable on the breath.126,127
The hypermethioninemias
Methionine is an essential sulphur containing amino acid. It is normally metabolised in a pathway resulting in cysteine. As discussed previously, DMS can be synthesised from transamination reactions of methionine. Thus hypermethioninaemia (HME) will accompany DMSE. HME is a sign, rather than a diagnosis, and represents several distinct clinical entities, both genetic and non-genetic.128 Many of the hypermethioninemias are syndromes presenting at birth and compromise more medically serious symptoms rather than malodour alone, such as brain demyelination.129 Methionine adenosyltransferase (MAT) I and III are the two isoenzymes that catalyse a vital stage in the methionine cycle, viz. the synthesis of S-adenosylmethionine from methionine. MAT I/III deficiency, secondary to mutations in the coding gene, MAT A1, have been described.130,131,132,133 MAT I/III deficiency may be implicated in isolated persistent hypermethioninaemia, an idiopathic cause of elevated methionine, in which hepatic MAT activity is normal and yet there are few serious clinical manifestations.134 Blood borne halitosis may still be a feature. Since DMSE accompanies HME, breath DMS can be used to screen for HME.135,136,137 A breath DMS level of 96 nM was reported in a patient with isolated persistent hypermethioninaemia secondary to MAT deficiency.122 Since DMSE can be caused by other conditions, elevated breath DMS is not specific for HME.
Foetor hepaticus
Portal hypertension and resultant portosystemic shunting can be caused by conditions like liver cirrhosis. Blood bypasses the portal circulation and hence volatiles that are absorbed from the GI tract are not immediately exposed to first pass metabolism. Traditionally, this foetor has been attributed to ketones and ammonia, however, more recent evidence suggests that DMS is the main odourant volatile, although ketones contribute to a lesser extent.138,139,140,141 In vitro experimentation with DMS solutions was reported to produce an odour very similar to that produced in fetor hepaticus.142 Mercaptans do not seem to directly cause hepatic encephalopathy, rather they just accumulate systemically.143,144 Awano et al. demonstrated elevated breath DMS in patients with various liver diseases.145 Interestingly, the same study showed correlation of elevated breath DMS with cerebrovascular disease. A secondary form of TMAU is also associated with liver disease, and blood borne TMA may even be contributory to the odour of foetor hepaticus.146,147 The character of foetor hepaticus has been varyingly described as musty, 'mousy' or sweetly faecal. There are also descriptions likening it to 'the smell of dead mice' or 'the breath of the dead'. Since DMS is the greatest contributor to the odour of foetor hepaticus, perhaps we can surmise that the in vivo expression and odour character of DMS is similar to that described for this condition. It is unlikely that DMSE and a related halitosis would be monosymptomatic for portal shunting, since this is frequently a late feature of decompensated liver cirrhosis, which comes with many other serious signs and symptoms. DMSE levels of 7-50 nM and breath DMS of 0.5-14.1 nM were demonstrated in patients with liver cirrhosis.142
Volatile foodstuffs
DMS, along with other VSC, contribute to the flavour of some foodstuffs and beverages.104,148,149,150,151 The metabolism of methionine was previously discussed, as were other DMS dietary precursors in plants. DMS in cabbage, among other VSC derivatives, have been investigated for their antimicrobial activity.152 Urinary DMS has been shown to be elevated after asparagus consumption,153 although DMS was not the major volatile produced.
Medications
Dimethylsulphoxide (DMSO) is used as vehicle for several drugs,154 as it is able to penetrate skin and other membranes without damage,155 but it can also be used as a therapeutic agent in its own right.156,157,158,159 It has uses as a topical agent, where interestingly its cutaneous administration can lead to a side effect of a garlic taste in the mouth.160 Whether this is due to blood borne presence of the DMS is unclear. A similar phenomenon is observed with disulphiram, where this is attributed to metabolism of the drug to carbon disulphide (CS2).161 CS2, a neurotoxin not thought to be endogenously produced, was also reported to be raised on the breath of patients with schizophrenia.162 DMSO is metabolised to dimethyl sulphone (DMSO2) and DMS, which is then subject to renal and pulmonary excretion.102,163,164,165,166,167
Nephropathic cystinosis, a rare autosomal recessive lysosomal storage condition, was previously a death sentence, however, cysteamine therapy now slows the progression of the disease and can prevent or delay the life threatening complications.168 Cysteamine removes the accumulated cystine from lysosome, however, an unfortunate side effect of this therapy is halitosis,169 which has been shown to be mostly due to presence of DMS in the blood. Hence, cystinotic patients on this therapy were shown to have elevated DMS in urine and breath.170
A case report documented elevated breath DMS in an asthmatic patient being treated with the anti-allergenic drug, suplatast tosilate,171 which appears to be metabolised to DMS.
Condition under study
The new metabolic condition that has potentially been identified by Tangerman and Winkle, awaits further investigation to identify the exact pathogenesis. Whether the dimethylsulphidemia is related to defects in the methionine cycle, or indeed another cause remains to be elucidated. The prevalence of the potential new condition has been estimated at 0.25-1.25% of the general population,32 which would make it more prevalent than other 'blood borne malodour' conditions previously identified. The extra-oral nature of the halitosis in these patients was deduced by demonstrating equal levels of DMS on the nose and mouth breath. Furthermore, blood DMS was shown to correlate perfectly with breath DMS. Experimentation with solutions of DMS was found to have an odour comparable to the character of halitosis in this group. Patients with this proposed metabolic condition are reported to have breath DMS levels of 0.5–2.5 nM91 and blood DMS levels of 10–80 n mol/l.32
Interestingly, a patient in this group showed significantly raised breath DMS 12 hours after consuming 12 glasses of beer. After this experiment, the patient in question was said to switch to drinking wine, and reported fewer complaints of bad breath.66
These findings could be interpreted with caution as they are based on a small sample, and are as yet uncorroborated by other researchers.
Discussion
'Systemic candidiasis syndrome'
There is a general belief among some section of the public that Candida overgrowth is a leading cause of halitosis, or even body malodour. It is interesting to note the evolution of a commercial industry revolving around the theory that a so called 'systemic candidiasis syndrome' (also termed 'candida hypersensitivity syndrome') is widely prevalent. This is a medically unrecognised condition and falls within the realms of pseudoscience and alternative medicine. To the author's knowledge, there is no published scientific paper that has proven Candida albicans or other yeasts as a cause of oral malodour or extra-oral halitosis. Indeed, one paper reported low Candida albicans carriage rates in halitosis patients.33 Candida is a normal oral commensal organism in a high percentage of the general population. Yeasts ferment carbohydrates to produce ethanol, which has an odour character described as 'strong alcoholic, ethereal or medical'.172 Whether high carriage rates of Candida spp. or even clinical evidence of oral candidiasis would produce a great enough concentration of ethanol or other metabolic by-products to create a malodour detectable by others is unknown. There is a feasible indirect, but not causative link between candidiasis and halitosis. Oral candidiasis frequently develops where there is xerostomia. Decreased salivary flow rates will reduce both the antimicrobial and mechanical cleansing action of saliva, resulting in increased growth of bacterial spp. in addition to Candida spp. The increased bacterial load could then lead to increased VSC levels and hence halitosis. In practice however, there does not seem to be any link between Candida and halitosis.
The so called 'systemic candidiasis syndrome', among its vast range of claimed symptoms, is described as giving a halitosis and/or body odour that is musty, 'mousy' or 'yeasty'. These particular odour descriptions are reminiscent of the odour character of DMS.
Blood borne halitosis may also cause other malodour complaints
Blood borne halitosis is caused by odourant volatiles present in the systemic circulation that are then transferred to the exhaled breath during pulmonary gas exchange. However, the blood goes just about everywhere. It is known that in other conditions, blood borne volatiles can be expressed via several routes of excretion, leading to the presence of volatiles in sweat, saliva and urine etc. This may cause multiple symptoms of malodour in these patients, for example, body odour, malodorous urine, etc. Thus, the concept of blood borne volatiles causing blood borne halitosis monosymptomatically may be an oversimplification. It is likely with many of these volatiles that pulmonary excretion is the main form of elimination, since this is a highly efficient system. The term blood borne body odour has been offered, but this again may be too constrictive and misleading. The author suggests the term 'blood borne malodour condition' to describe these conditions and to reflect the multiple malodour complaints that they may be capable of triggering.
There are logistical problems involved in breath analysis,173 and its availability may be limited in some areas. The relevance of breath analysis in the management of halitosis is undisputed, but it may be useful to perform urinalysis or blood biochemistry in selected cases, eg, where blood borne halitosis is suspected and there is a failure of standard therapy for intra-oral halitosis to control the symptom which cannot be better explained. These alternative routes may be more available and familiar to clinicians. Further research would be needed to validate these methods as being predictive for malodour symptoms and if so at what threshold concentration in the blood is required to create symptoms.
Trimethylaminuria
Trimethylaminuria (TMAU) has dominated researchers' interest into blood borne malodour conditions. The implicated enzyme pathway is the xenobiotic metabolising enzyme (XME) group, flavin containing mono-oxygenases (FMO), particularly FMO3. FMO has been implicated as being of perhaps equal importance to CYP450.174 Evidence suggests that FMO is also involved in the metabolism of certain drugs.175,176,177,178 The main role of the FMO pathway appears to be as a scavenger system with a low specificity, carrying out oxidation of thousands of substrates, most notably nucleophilic amines and sulphides.174 One of these substrates is trimethylamine (TMA), which has a fish like odour, becoming ammoniacal at higher concentrations. Genetic mutations of FMO3 that result in reduced efficiency when acting on the volatile TMA, (normally N-oxidised to trimethylamine N-oxide), leading to presence of TMA in the systemic circulation. This is said to create a fish-like blood borne body odour and halitosis.179 Secondary forms of TMAU, where FMO efficiency may be at normal levels and an increase in the production of volatiles may overload the pathway, creating a mismatch between the substrate work load and the enzymes' ability to act on them.180 There also appears to be a lack of consensus regarding the ideal diagnostic threshold.
Reported incidence of heterozygous (carrier) TMAU incidence has been estimated at 0.5-1% in the white British population.95,181 Patients with the heterozygote phenotype are thought by some to be 'at risk' of having their already compromised enzyme pathway overloaded easily,180 possibly producing a transient presence of blood borne volatiles and an intermittent malodour symptom. Evidently, the number of patients with symptomatic TMAU is likely lower than these figures. This notion is supported when one looks at reported data from a major TMAU testing centre for over a decade. The number of patients receiving a positive diagnosis is low, around 30%. If we assume that a high percentage of these patients have genuine malodour symptoms (since they have been referred by a clinician for the test), then it appears that TMAU does not explain the majority of patients with suspected blood borne malodour conditions.
Anecdotally, only a minority of TMAU positive patients and patients who test negatively for TMAU, display the classically described fish odour, instead a range of odours are reported, such as faeculant body odour and halitosis. It is currently unknown whether other odourant volatiles normally dealt with by FMO3 could become elevated systemically as a result of dysfunction or overload of this enzyme pathway. It is interesting to note that FMO may be at least partly responsible for metabolism of DMS,117,182,183
Conclusion
Frequently extra-oral halitosis, particularly blood borne halitosis, represents a far greater diagnostic and therapeutic challenge than 'straightforward' intra-oral halitosis. It has been suggested that a general lack of practitioner awareness is leading to ineffective management, and long delays in diagnosis of potentially treatable cases, possibly increasing detrimental psychosocial implications. This is a rapidly developing area in halitology and it is anticipated that diagnosis and management of blood borne malodour conditions will soon be better understood, allowing more effective management to address unmet patient needs.
References
Yaegaki K, Brunette D M, Tangerman A et al. Standardization of clinical protocols in oral malodour research. J Breath Res 2012; 6: 017101.
Söder B, Johansson B, Söder P O . The relation between foetor ex ore, oral hygiene and periodontal disease. Swed Dent J 2000; 24: 73–82.
Liu X N, Shinada K, Chen X C, Zhang B X, Yaegaki K, Kawaguchi Y . Oral malodour-related parameters in the Chinese general population. J Clin Periodontol 2006; 33: 31–36.
Huang X, Li X, Fan X, Liu H . [Levels of volatile sulphur compounds and the analysis of related factors in oral cavities of 384 health subjects in Chengdu]. Hua Xi Kou Qiang Yi Xue Za Zhi 2002; 20: 380–382.
Bornstein M M, Stocker B L, Seemann R, Bürgin W B, Lussi A . Prevalence of halitosis in young male adults: a study in swiss army recruits comparing self-reported and clinical data. J Periodontol 2009; 80: 24–31.
Zürcher A, Filippi A . Findings, diagnoses and results of a halitosis clinic over a seven year period. Schweiz Monatsschr Zahnmed 2012; 122: 205–216.
Loesche W J, Kazor C . Microbiology and treatment of halitosis. Periodontol 2000 2002; 28: 256–279.
Rayman S, Almas K . Halitosis among racially diverse populations: an update. Int J Dent Hyg 2008; 6: 2–7.
Adeyemi B F, Kolude B M, Arigbede A O . Attitude and perception of mouth odour in 213 respondents. Niger Postgrad Med J 2012; 19: 97–101.
Hoover K C . Smell with inspiration: the evolutionary significance of olfaction. Am J Phys Anthropol 2010; 143 (Suppl 51): 63–74.
Yeshurun Y, Sobel N . An odour is not worth a thousand words: from multidimensional odours to unidimensional odour objects. Annu Rev Psychol 2010; 61: 219–241, C1–5.
Roberts S C, Kralevich A, Ferdenzi C et al. Body odor quality predicts behavioural attractiveness in humans. Arch Sex Behav 2011; 40: 1111–1117.
Sergeant M J . Female perception of male body odour. Vitam Horm 2010; 83: 25–45.
Craig Roberts S, Little A C, Lyndon A, Roberts J, Havlicek J, Wright R L . Manipulation of body odour alters men's self-confidence and judgements of their visual attractiveness by women. Int J Cosmet Sci 2009; 31: 47–54.
Demattè M L, Osterbauer R, Spence C . Olfactory cues modulate facial attractiveness. Chem Senses 2007; 32: 603–610.
Grammer K, Fink B, Neave N . Human pheromones and sexual attraction. Eur J Obstet Gynecol Reprod Biol 2005; 118: 135–142.
Moshkin M, Litvinova N, Litvinova E A, Bedareva A, Lutsyuk A, Gerlinskaya L . Scent recognition of infected status in humans. J Sex Med 2011; 9: 3211–3218.
Prugnolle F, Lefèvre T, Renaud F, Møller A P, Missé D, Thomas F . Infection and body odours: evolutionary and medical perspectives. Infect Genet Evol 2009; 9: 1006–1009.
DeWall C N, Deckman T, Pond R S Jr, Bonser I . Belongingness as a core personality trait: how social exclusion influences social functioning and personality expression. J Pers 2011; 79: 1281–1314.
Zwolinski J . Psychological and neuroendocrine reactivity to ostracism. Aggress Behav 2012; epub ahead of print.
Kelly M, McDonald S, Rushby J . All alone with sweaty palms - physiological arousal and ostracism. Int J Psychophysiol 2012; 83: 309–314.
McKeown L . Social relations and breath odour. Int J Dent Hyg 2003; 1: 213–217.
Zaitsu T, Ueno M, Shinada K, Wright F A, Kawaguchi Y . Social anxiety disorder in genuine halitosis patients. Health Qual Life Outcomes 2011; 9: 94.
Nardi G M, Forabosco A, Forabosco G, Musciotto A, Campisi G, Grandi T . Halitosis: a stomatological and psychological issue. Minerva Stomatol 2009; 58: 435–444.
Settineri S, Mento C, Gugliotta S C et al. Self-reported halitosis and emotional state: impact on oral conditions and treatments. Health Qual Life Outcomes 2010; 8: 34.
Coil J M, Yaegaki K, Matsuo T, Miyazaki H . Treatment needs (TN) and practical remedies for halitosis. Int Dent J 2002; 52 (Suppl 3): 187–191.
Bollen C M, Beikler T . Halitosis: the multidisciplinary approach. Int J Oral Sci 2012; 4: 55–63.
Seemann R, Bizhang M, Djamchidi C, Kage A, Nachnani S . The proportion of pseudo-halitosis patients in a multidisciplinary breath malodour consultation. Int Dent J 2006; 56: 77–81.
Filippi A, Müller N . [Real and psychological halitosis - findings, diagnoses and outcomes of a halitosis clinic]. Schweiz Monatsschr Zahnmed 2006; 116: 129–135.
Delanghe G, Bollen C, Desloovere C . [Halitosis-foetor ex ore]. Laryngorhinootologie 1999; 78: 521–524.
Uguru C, Umeanuka O, Uguru N P, Adigun O, Edafioghor O . The delusion of halitosis: experience at an eastern Nigerian tertiary hospital. Niger J Med 2011; 20: 236–240.
Tangerman A, Winkel E G . Intra-and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J Clin Periodontol 2007; 34: 748–755.
Ben-Aryeh H, Horowitz G, Nir D, Laufer D . Halitosis: an interdisciplinary approach. Am J Otolaryngol 1998; 19: 8–11.
Quirynen M, Dadamio J, Van den Velde S et al. Characteristics of 2000 patients who visited a halitosis clinic. J Clin Periodontol 2009; 36: 970–975.
Winkel E G . Halitosis control. In Lindhe J, Lang N P, Karring T (eds) Clinical periodontology and implant dentistry. 5th edn. pp 1324–1340. Oxford: Blackwell Munksgaard, 2008.
Quirynen M, Van den Velde S, Vandekerckhove B, Dadamio J . Oral malodour. In Newman M G, Takei H H, Klokkevold P R, Carranza F A (eds) Carranza's clinical periodontology. 11th edn. pp 331–338. St. Louis, Mo: Elsevier/Saunders, 2012.
Tangerman A, Winkel E G . The portable gas chromatograph OralChroma™: a method of choice to detect oral and extra-oral halitosis. J Breath Res 2008; 2: 017010.
Preti G, Clark L, Cowart B J et al. Non-oral etiologies of oral malodour and altered chemosensation. J Periodontol 1992; 63: 790–796.
Gupta P V . Bad taste. In Gupta P V Differential diagnosis of dental diseases. New Delhi: Jaypee Brothers Medical Publishers, 2008.
Scully C . Sensory and motor changes and taste abnormalities. In Scully C Oral and maxillofacial medicine: the basis of diagnosis and treatment. 2nd edn. Chapter 13. Edinburgh: Churchill Livingstone, 2008.
Heckmann J G, Lang C J . Neurological causes of taste disorders. Adv Otorhinolaryngol 2006; 63: 255–264.
Mott A E, Grushka M, Sessle B J . Diagnosis and management of taste disorders and burning mouth syndrome. Dent Clin North Am 1993; 37: 33–71.
Hummel T, Landis B N, Hüttenbrink K B . Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg 2011; 10: Doc04.
Leopold D . Distortion of olfactory perception: diagnosis and treatment. Chem Senses 2002; 27: 611–615.
Landis B N, Burkhard P R . Phantosmias and Parkinson disease. Arch Neurol 2008; 65: 1237–1239.
Ayad T, Khoueir P, Saliba I, Moumdjian R . Cacosmia secondary to an olfactory groove meningioma. J Otolaryngol 2007; 36: E21–23.
Nordin S, Broman D A, Olofsson J K, Wulff M . A longitudinal descriptive study of self-reported abnormal smell and taste perception in pregnant women. Chem Senses 2004; 29: 391–402.
Müller A, Landis B N, Platzbecker U, Holthoff V, Frasnelli J, Hummel T . Severe chemotherapy-induced parosmia. Am J Rhinol 2006; 20: 485–486.
Rosenberg M, Kozlovsky A, Gelernter I et al. Self-estimation of oral malodour. J Dent Res 1995; 74: 1577–1582.
American Psychiatric Association DSM-5 Development. Olfactory reference syndrome (proposed for section III of the DSM-5). Arlington, Va: American Psychiatric Association, 2012.
Falcão D P, Vieira C N, Batista de Amorim R F . Breaking paradigms: a new definition for halitosis in the context of pseudo-halitosis and halitophobia. J Breath Res 2012; 6: 017105.
Romano F, Pigella E, Guzzi N, Aimetti M . Patients' self-assessment of oral malodour and its relationship with organoleptic scores and oral conditions. Int J Dent Hygiene 2010; 8: 41–46.
Tangerman A . Halitosis in medicine: a review. Int Dent J 2002; 52 (Suppl 3): 201–206.
Scully C, Greenman J . Halitology (breath odour: aetiopathogenesis and management). Oral Dis 2012; 18: 333–345.
Richter J L . Diagnosis and treatment of halitosis. Compend Contin Educ Dent 1996; 17: 370–372.
Washio J, Sato T, Koseki T, Takahashi N . Hydrogen sulphide-producing bacteria in tongue biofilm and their relationship with oral malodour. J Med Microbiol 2005; 54: 889–895.
Feller L, Blignaut E . Halitosis: a review. SADJ 2005; 60: 17–19.
Pratibha P K, Bhat K M, Bhat G S . Oral malodour: a review of the literature. J Dent Hyg 2006; 80: 8.
Cortelli J R, Barbosa M D, Westphal M A . Halitosis: a review of associated factors and therapeutic approach. Braz Oral Res 2008; 22 (Suppl 1): 44–54.
Lee P P, Mak W Y, Newsome P . The aetiology and treatment of oral halitosis: an update. Hong Kong Med J 2004; 10: 414–418.
van den Broek A M, Feenstra L, de Baat C . A review of the current literature on management of halitosis. Oral Dis 2008; 14: 30–39.
Armstrong B L, Sensat M L, Stoltenberg J L . Halitosis: a review of current literature. J Dent Hyg 2010; 84: 65–74.
Yaegaki K, Coil J M . Examination, classification, and treatment of halitosis; clinical perspectives. J Can Dent Assoc 2000; 66: 257–261.
Fedorowicz Z, Aljufairi H, Nasser M, Outhouse T L, Pedrazzi V . Mouthrinses for the treatment of halitosis. Cochrane Database Syst Rev 2008; (4): CD006701.
Outhouse T L, Al-Alawi R, Fedorowicz Z, Keenan J V . Tongue scraping for treating halitosis. Cochrane Database Syst Rev 2006; (2): CD005519.
Tangerman A, Winkel E G . Extra-oral halitosis: an overview. J Breath Res 2010; 4: 017003.
Attia E L, Marshall K G . Halitosis. Can Med Assoc J 1982; 126: 1281–1285.
Katz J, Shenkman A, Stavropoulos F, Melzer E . Oral signs and symptoms in relation to disease activity and site of involvement in patients with inflammatory bowel disease. Oral Dis 2003; 9: 34–40.
Kim J G, Kim Y J, Yoo S H et al. Halimeter ppb levels as the predictor of erosive gastroesophageal reflux disease. Gut Liver 2010; 4: 320–325.
Katsinelos P, Tziomalos K, Chatzimavroudis G et al. Eradication therapy in Helicobacter pylori-positive patients with halitosis: long-term outcome. Med Princ Pract 2007; 16: 119–123.
Porter S R . Diet and halitosis. Curr Opin Clin Nutr Metab Care 2011; 14: 463–468.
Moshkowitz M, Horowitz N, Leshno M, Halpern Z . Halitosis and gastroesophageal reflux disease: a possible association. Oral Dis 2007; 13: 581–585.
Norfleet R G . Helicobacter halitosis. J Clin Gastroenterol 1993; 16: 274.
Tiomny E, Arber N, Moshkowitz M, Peled Y, Gilat T . Halitosis and Helicobacter pylori. A possible link? J Clin Gastroenterol 1992; 15: 236–237.
Ierardi E, Amoruso A, La Notte T et al. Halitosis and Helicobacter pylori: a possible relationship. Dig Dis Sci 1998; 43: 2733–2737.
Adler I, Denninghoff V C, Alvarez M I, Avagnina A, Yoshida R, Elsner B . Helicobacter pylori associated with glossitis and halitosis. Helicobacter 2005; 10: 312–317.
Lee J S, Kwon K A, Jung H S, Kim J H, Hahm K B . Korea red ginseng on Helicobacter pylori-induced halitosis: newer therapeutic strategy and a plausible mechanism. Digestion 2009; 80: 192–199.
Struch F, Schwahn C, Wallaschofski H et al. Self-reported halitosis and gastro-esophageal reflux disease in the general population. J Gen Intern Med 2008; 23: 260–266.
Suzuki N, Yoneda M, Naito T et al. Detection of Helicobacter pylori DNA in the saliva of patients complaining of halitosis. J Med Microbiol 2008; 57: 1553–1559.
Hoshi K, Yamano Y, Mitsunaga A, Shimizu S, Kagawa J, Ogiuchi H . Gastrointestinal diseases and halitosis: association of gastric Helicobacter pylori infection. Int Dent J 2002; 52 (Suppl 3): 207–211.
Kinberg S, Stein M, Zion N, Shaoul R . The gastrointestinal aspects of halitosis. Can J Gastroenterol 2010; 24: 552–556.
Kislig K, Wilder-Smith C H, Bornstein M M, Lussi A, Seemann R . Halitosis and tongue coating in patients with erosive gastroesophageal reflux disease versus nonerosive gastroesophageal reflux disease. Clin Oral Investig 2013; 17: 159–165.
Rosenberg M . Clinical assessment of bad breath: current concepts. J Am Dent Assoc 1996; 127: 475–482.
Ng D K, Chow P Y, Kwok K L . Halitosis and the nose. Hong Kong Med J 2005; 11: 71–72.
Motta L J, Bachiega J C, Guedes C C, Laranja L T, Bussadori S K . Association between halitosis and mouth breathing in children. Clinics (Sao Paulo) 2011; 66: 939–942.
Bollen C M, Rompen E H, Demanez J P . [Halitosis: a multidisciplinary problem]. Rev Med Liege 1999; 54: 32–36.
Bollen C M, Beikler T . Halitosis: the multidisciplinary approach. Int J Oral Sci 2012; 4: 55–63.
Ghoshal U C . How to interpret hydrogen breath tests. J Neurogastroenterol Motil 2011; 17: 312–317.
Ghoshal U C, Ghoshal U, Das K, Misra A . Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol 2006; 25: 6–10.
Simrén M, Stotzer P O . Use and abuse of hydrogen breath tests. Gut 2006; 55: 297–303.
Tangerman A . Measurement and biological significance of the volatile sulphur compounds hydrogen sulphide, methanethiol and dimethyl sulphide in various biological matrices. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 3366–3377.
Danzl D F . Flatology. J Emerg Med 1992; 10: 79–88.
Suarez F L, Springfield J, Levitt M D . Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut 1998; 43: 100–104.
Cashman J R, Camp K, Fakharzadeh S S et al. Biochemical and clinical aspects of the human flavin-containing monooxygenase form 3 (FMO3) related to trimethylaminuria. Curr Drug Metab 2003; 4: 151–170.
Phillips I R, Shephard E A . Trimethylaminuria. In Pagon R A, Bird T D, Dolan C R, Stephens K, Adam M P (eds) GeneReviews™ (online). Washington: University of Washington, 2007. Online article available at http://www.ncbi.nlm.nih.gov/books/NBK1103/ (accessed March 2013).
Binzak B A, Wevers R A, Moolenaar S H et al. Cloning of dimethylglycine dehydrogenase and a new human inborn error of metabolism, dimethylglycine dehydrogenase deficiency. Am J Hum Genet 2001; 68: 839–847.
Delanghe G, Ghyselen J, Feenstra L, van Steenberghe D . Experiences of a Belgian multidisciplinary breath odour clinic. Acta Otorhinolaryngol Belg 1997; 51: 43–48.
Clark G T, Nachnani S, Messadi D V . Detecting and treating oral and nonoral malodours. J Calif Dent Assoc 1997; 25: 133–144.
Dimethyl sulphide, Organoleptics (online). Oak Creek, WI: The Good Scents Company. Online informtation available at http://www.thegoodscentscompany.com/data/rw1022341.html (accessed March 2013).
Knasko S C . Ambient odor's effect on creativity, mood, and perceived health. Chem Senses 1992; 17: 27–35.
Terazawa K, Mizukami K, Wu B, Takatori T . Fatality due to inhalation of dimethyl sulphide in a confined space: a case report and animal experiments. Int J Legal Med 1991; 104: 141–144.
National Institutes of Health. Hazardous substances data bank fact sheet. Maryland: US National Library of Medicine. Online information available at http://www.nlm.nih.gov/pubs/factsheets/hsdbfs.html (accessed March 2013).
Otte M L, Wilson G, Morris J T, Moran B M . Dimethylsulphoniopropionate (DMSP) and related compounds in higher plants. J Exp Bot 2004; 55: 1919–1925.
Pimenta M J, Kaneta T, Larondelle Y, Dohmae N, Kamiya Y . S-adenosyl-L-methionine: L-methionine S-methyltransferase from germinating barley. Purification and localization. Plant Physiol 1998; 118: 431–438.
Blom H J, Boers G H, van den Elzen J P, Gahl W A, Tangerman A . Transamination of methionine in humans. Clin Sci (Lond) 1989; 76: 43–49.
Weisiger R A, Pinkus L M, Jakoby W B . Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulphide. Biochem Pharmacol 1980; 29: 2885–2887.
Blom H J, van den Elzen J P, Yap S H, Tangerman A . Methanethiol and dimethylsulphide formation from 3-methylthiopropionate in human and rat hepatocytes. Biochim Biophys Acta 1988; 972: 131–136.
Mhatre S S, Chetty K G, Pradhan D S . Uncoupling of oxidative phosphorylation in rat liver mitochondria following the administration of dimethyl sulphoxide. Biochem Biophys Res Commun 1983; 110: 325–331.
Antunes L C, Buckner M M, Auweter S D, Ferreira R B, Lolić P, Finlay B B . Inhibition of Salmonella host cell invasion by dimethyl sulphide. Appl Environ Microbiol 2010; 76: 5300–5304.
Horinouchi M, Kasuga K, Nojiri H, Yamane H, Omori T . Cloning and characterization of genes encoding an enzyme which oxidizes dimethyl sulphide in Acinetobacter sp. strain 20B. FEMS Microbiol Lett 1997; 155: 99–105.
Weiner J H, Rothery R A, Sambasivarao D, Trieber C A . Molecular analysis of dimethylsulphoxide reductase: a complex iron-sulphur molybdoenzyme of Escherichia coli. Biochim Biophys Acta 1992; 1102: 1–18.
Suarez F, Furne J, Springfield J, Levitt M . Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol 1997; 272: G1028–1033.
Suarez F, Furne J, Springfield J, Levitt M . Production and elimination of sulphur-containing gases in the rat colon. Am J Physiol 1998; 274: G727–733.
Levitt M D, Furne J, Springfield J, Suarez F, DeMaster E . Detoxification of hydrogen sulphide and methanethiol in the cecal mucosa. J Clin Invest 1999; 104: 1107–1114.
Furne J, Springfield J, Koenig T, DeMaster E, Levitt M D . Oxidation of hydrogen sulphide and methanethiol to thiosulphate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol 2001; 62: 255–259.
Porro C S, Sutcliffe M J, de Visser S P . Quantum mechanics/molecular mechanics studies on the sulphoxidation of dimethyl sulphide by compound I and compound 0 of cytochrome P450: which is the better oxidant? J Phys Chem A 2009; 113: 11635–11642.
Bach R D . Role of the somersault rearrangement in the oxidation step for flavin monooxygenases (FMO). A comparison between FMO and conventional xenobiotic oxidation with hydroperoxides. J Phys Chem A 2011; 115: 11087–11100.
Williams K I, Burstein S H, Layne D S . Metabolism of dimethyl sulphide, dimethyl sulphoxide, and dimethyl sulphone in the rabbit. Arch Biochem Biophys 1966; 117: 84–87.
Susman J L, Hornig J F, Thomae S C, Smith R P . Pulmonary excretion of hydrogen sulphide, methanethiol, dimethyl sulphide and dimethyl disulphide in mice. Drug Chem Toxicol 1978; 1: 327–338.
Clark W A, Salsbury R L . Dimethyl sulphide in milk of lactating dairy cows fed various sulphur compounds. J Dairy Sci 1980; 63: 375–378.
Tangerman A, Meuwese-Arends M T, van Tongeren J H . New methods for the release of volatile sulphur compounds from human serum: its determination by Tenax trapping and gas chromatography and its application in liver diseases. J Lab Clin Med 1985; 106: 175–182.
Gahl W A, Bernardini I, Finkelstein J D et al. Transsulphuration in an adult with hepatic methionine adenosyltransferase deficiency. J Clin Invest 1988; 81: 390–397.
Awano S, Takata Y, Soh I et al. Correlations between health status and OralChroma™-determined volatile sulphide levels in mouth air of the elderly. J Breath Res 2011; 5: 046007.
Ansai T, Awano S, Soh I et al. Associations among hair loss, oral sulphur-containing gases, and gastrointestinal and metabolic linked diseases in Japanese elderly men: pilot study. BMC Public Health 2009; 9: 82.
Barker M, Hengst M, Schmid J et al. Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur Respir J 2006; 27: 929–936.
Goyer N . Evaluation of occupational exposure to sulphur compounds in paper pulp kraft mills. Am Ind Hyg Assoc J 1990; 51: 390–394.
Jäppinen P, Kangas J, Silakoski L, Savolainen H . Volatile metabolites in occupational exposure to organic sulphur compounds. Arch Toxicol 1993; 67: 104–106.
Mudd S H . Hypermethioninemias of genetic and non-genetic origin: a review. Am J Med Genet C Semin Med Genet 2011; 157: 3–32.
Chamberlin M E, Ubagai T, Mudd S H, Wilson W G, Leonard J V, Chou J Y . Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. J Clin Invest 1996; 98: 1021–1027.
Ubagai T, Lei K J, Huang S, Mudd S H, Levy H L, Chou J Y . Molecular mechanisms of an inborn error of methionine pathway. Methionine adenosyltransferase deficiency. J Clin Invest 1995; 96: 1943–1947.
Chamberlin M E, Ubagai T, Mudd S H, Levy H L, Chou J Y . Dominant inheritance of isolated hypermethioninaemia is associated with a mutation in the human methionine adenosyltransferase 1A gene. Am J Hum Genet 1997; 60: 540–546.
Chamberlin M E, Ubagai T, Mudd S H et al. Methionine adenosyltransferase I/III deficiency: novel mutations and clinical variations. Am J Hum Genet 2000; 66: 347–355.
Hazelwood S, Bernardini I, Shotelersuk V et al. Normal brain myelination in a patient homozygous for a mutation that encodes a severely truncated methionine adenosyltransferase I/III. Am J Med Genet 1998; 75: 395–400.
Mudd S H, Levy H L, Tangerman A et al. Isolated persistent hypermethioninaemia. Am J Hum Genet 1995; 57: 882–892.
Kaji H, Hisamura M, Saito N, Murao M . Biochemical aspect of dimethyl sulphide breath test in the studies on methionine metabolism. Res Commun Chem Pathol Pharmacol 1981; 32: 515–523.
Kaji H, Hisamura M, Saito N et al. Clinical application of breath analysis for dimethyl sulphide following ingestion of DL-methionine. Clin Chim Acta 1979; 93: 377–380.
Kaji H, Saito N, Hisamura M et al. Nutritional aspect of methionine isomers studied by pulmonary exhalation of dimethyl sulphide and urinary excretion of alpha-keto-gamma-methiolbutyrate in humans. Jpn J Med 1983; 22: 106–111.
Van den Velde S, Nevens F, Van Hee P, van Steenberghe D, Quirynen M . GC-MS analysis of breath odour compounds in liver patients. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 875: 344–348.
Chen S, Zieve L, Mahadevan V . Mercaptans and dimethyl sulphide in the breath of patients with cirrhosis of the liver. Effect of feeding methionine. J Lab Clin Med 1970; 75: 628–635.
Kaji H, Hisamura M, Saito N, Murao M . Evaluation of volatile sulphur compounds in the expired alveolar gas in patients with liver cirrhosis. Clin Chim Acta 1978; 85: 279–284.
Kaji H, Hisamura M, Saito N, Murao M . Gas chromatographic determination of volatile sulphur compounds in the expired alveolar air in hepatopathic subjects. J Chromatogr 1978; 145: 464–468.
Tangerman A, Meuwese-Arends M T, Jansen J B . Cause and composition of foetor hepaticus. Lancet 1994; 343: 483.
Al Mardini H, Bartlett K, Record C O . Blood and brain concentrations of mercaptans in hepatic and methanethiol induced coma. Gut 1984; 25: 284–290.
Al Mardini H, Leonard J, Bartlett K, Lloyd S, Record C O . Effect of methionine loading and endogenous hypermethioninaemia on blood mercaptans in man. Clin Chim Acta 1988; 176: 83–89.
Awano S, Ansai T, Takata Y et al. Relationship between volatile sulphur compounds in mouth air and systemic disease. J Breath Res 2008; 2: 017012.
Fernández M S, Gutierrez C, Vila J J et al. Congenital intrahepatic portocaval shunt associated with trimethylaminuria. Pediatr Surg Int 1997; 12: 196–197.
Mitchell S, Ayesh R, Barrett T, Smith R . Trimethylamine and foetor hepaticus. Scand J Gastroenterol 1999; 34: 524–528.
Scherb J, Kreissl J, Haupt S, Schieberle P . Quantitation of S-methylmethionine in raw vegetables and green malt by a stable isotope dilution assay using LC-MS/MS: comparison with dimethyl sulphide formation after heat treatment. J Agric Food Chem 2009; 57: 9091–9096.
Engel E, Baty C, Le Corre D, Souchon I, Martin N . Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J Agric Food Chem 2002; 50: 6459–6467.
Scarlata C J, Ebeler S E . Headspace solid-phase microextraction for the analysis of dimethyl sulphide in beer. J Agric Food Chem 1999; 47: 2505–2508.
Segurel M A, Razungles A J, Riou C, Salles M, Baumes R L . Contribution of dimethyl sulphide to the aroma of Syrah and Grenache Noir wines and estimation of its potential in grapes of these varieties. J Agric Food Chem 2004; 52: 7084–7093.
Kyung K H, Fleming H P . Antimicrobial activity of sulphur compounds derived from cabbage. J Food Prot 1997; 60: 67–71.
Waring R H, Mitchell S C, Fenwick G R . The chemical nature of the urinary odour produced by man after asparagus ingestion. Xenobiotica 1987; 17: 1363–1371.
Marren K . Dimethyl sulphoxide: an effective penetration enhancer for topical administration of NSAIDs. Phys Sportsmed 2011; 39: 75–82.
Williams A C, Barry B W . Penetration enhancers. Adv Drug Deliv Rev 2004; 56: 603–618.
Shirley S W, Stewart B H, Mirelman S . Dimethyl sulphoxide in treatment of inflammatory genitourinary disorders. Urology 1978; 11: 215–220.
Gaspar M, Bovaira M, Carrera-Hueso F J, Querol M, Jiménez A, Moreno L . Efficacy of a topical treatment protocol with dimethyl sulphoxide 50% in type 1 complex regional pain syndrome. Farm Hosp 2012. 36: 386–391.
Iwasaki T, Hamano T, Aizawa K, Kobayashi K, Kakishita E . A case of pulmonary amyloidosis associated with multiple myeloma successfully treated with dimethyl sulphoxide. Acta Haematol 1994; 91: 91–94.
French L M, Bhambore N . Interstitial cystitis/painful bladder syndrome. Am Fam Physician 2011; 83: 1175–1181.
Dimethylsulphoxide. In Novak K M (ed) Drug facts and comparisons. 56th ed. St. Louis: Wolters Kluwer Health, 2002.
O'Reilly R A, Motley C H . Breath odour after disulphiram. JAMA 1977; 238: 2600.
Phillips M, Sabas M, Greenberg J . Increased pentane and carbon disulphide in the breath of patients with schizophrenia. J Clin Pathol 1993; 46: 861–864.
Layman D L, Jacob S W . The absorption, metabolism and excretion of dimethyl sulphoxide by rhesus monkeys. Life Sci 1985; 37: 2431–2437.
Swanson B N, Mojaverian P, Boppana V K . Inhibition of sulindac metabolism by dimethyl sulphoxide in the rat. J Toxicol Environ Health 1983; 12: 213–222.
Wood P M . The redox potential for dimethyl sulphoxide reduction to dimethyl sulphide: evaluation and biochemical implications. FEBS Lett 1981; 124: 11–14.
Kocsis J J, Harkaway S, Snyder R . Biological effects of the metabolites of dimethyl sulphoxide. Ann N Y Acad Sci 1975; 243: 104–109.
Jeffery E H, Arndt K, Haschek W M . Mechanism of inhibition of hepatic bioactivation of paracetamol by dimethyl sulphoxide. Drug Metabol Drug Interact 1988; 6: 413–24.
Kleta R, Gahl W A . Pharmacological treatment of nephropathic cystinosis with cysteamine. Expert Opin Pharmacother 2004; 5: 2255–2262.
Besouw M, Blom H, Tangerman A, de Graaf-Hess A, Levtchenko E . The origin of halitosis in cystinotic patients due to cysteamine treatment. Mol Genet Metab 2007; 91: 228–233.
Gahl W A, Ingelfinger J, Mohan P, Bernardini I, Hyman P E, Tangerman A . Intravenous cysteamine therapy for nephropathic cystinosis. Pediatr Res 1995; 38: 579–584.
Murata T, Fujiyama Y, Yamaga T, Miyazaki H . Breath malodor in an asthmatic patient caused by side-effects of medication: a case report and review of the literature. Oral Dis 2003; 9: 273–276.
Ethanol, organoleptics. Oak Creek, WI: The Good Scents Company. Online information available at http://www.thegoodscentscompany.com/data/rw1000511.html (accessed March 2013).
Phillips M . Detection of volatile organic compounds in breath. In Marczin N, Yacoub M H (eds) Disease markers in exhaled breath: basic mechanisms and clinical applications. Oxford: NATO Science Series, IOS Press, 2002.
Mitchell S C . Flavin mono-oxygenase (FMO) - the 'other' oxidase. Curr Drug Metab 2008; 9: 280–284.
Xie G, Wong C C, Cheng K W, Huang L, Constantinides P P, Rigas B . Regioselective oxidation of phospho-NSAIDs by human cytochrome P450 and flavin monooxygenase isoforms: implications for their pharmacokinetic properties and safety. Br J Pharmacol 2012; 167: 222–232.
Krueger S K, Williams D E . Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther 2005; 106: 357–387.
Cashman J R . Human flavin-containing monooxygenase: substrate specificity and role in drug metabolism. Curr Drug Metab 2000; 1: 181–191.
Cashman J R, Park S B, Berkman C E, Cashman L E . Role of hepatic flavin-containing monooxygenase 3 in drug and chemical metabolism in adult humans. Chem Biol Interact 1995; 96: 33–46.
Mitchell S C . Trimethylaminuria (fish-odour syndrome) and oral malodour. Oral Dis 2005; 11 (Suppl 1): 10–13.
Mitchell S C . Trimethylaminuria: susceptibility of heterozygotes. Lancet 1999; 354: 2164–2165.
Sabourin P J, Hodgson E . Characterization of the purified microsomal FAD-containing monooxygenase from mouse and pig liver. Chem Biol Interact 1984; 51: 125–139.
Poulsen L L . Organic sulphur substrates for the microsomal flavin-containing monooxygenase. In Hodgson E, Bend J R, Philpot R M (eds) Rev Biochem Toxicol. North Holland: Elsevier Press, 1981.
Mitchell S C, Zhang A Q, Barrett T, Ayesh R, Smith R L. Studies on the discontinuous N-oxidation of trimethylamine among Jordanian, Ecuadorian and New Guinean populations. Pharmacogenetics 1997; 7: 45–50.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Harvey-Woodworth, C. Dimethylsulphidemia: the significance of dimethyl sulphide in extra-oral, blood borne halitosis. Br Dent J 214, E20 (2013). https://doi.org/10.1038/sj.bdj.2013.329
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2013.329
This article is cited by
-
Halitosis: etiology, prevention, and the role of microbiota
Clinical Oral Investigations (2023)
-
The tongue biofilm metatranscriptome identifies metabolic pathways associated with the presence or absence of halitosis
npj Biofilms and Microbiomes (2022)
-
Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis
Nature Genetics (2018)
-
Halitosis: a new definition and classification
British Dental Journal (2014)