Key Points
-
A range of 'loose' (non-bound to the muco-periosteal complex) soft tissue pathologies are amenable to treatment with surgical lasers.
-
Laser-assisted surgery should follow strict guidelines of 'best practice' approach, as employed in conventional therapies.
-
Laser-assisted surgical sites heal by secondary intention.
-
In comparison to scalpel incisions, laser cutting heals no faster, but may be more predictable through the control of bleeding and bacterial contamination.
Key Points
Lasers in dentistry
-
1
Introduction, history of lasers and laser light production
-
2
Laser-tissue interaction
-
3
Low-level laser use in dentistry
-
4
Lasers and soft tissue: 'loose' soft tissue surgery
-
5
Lasers and soft tissue: 'fixed' soft tissue surgery
-
6
Lasers and soft tissue: periodontal therapy
-
7
Surgical laser use in implantology and endodontics
-
8
Surgical lasers and hard dental tissue
-
9
Laser regulation and safety in general dental practice
Abstract
Oral soft tissue is composed of collagen, water, pigmented connective tissue, blood and lymphatic vessels. In that each may be considered target chromophores, all commercially available laser wavelengths in dentistry will interact with these component elements to a greater or lesser extent. What is of prime importance is that consideration is given to the predominant chromophore in any target tissue and the laser wavelength matched to achieve maximum absorption of light energy. Laser surgery can offer haemostasis, fewer post-operative complications and greater patient acceptance. This article examines the common 'loose' soft tissue management procedures in general dental practice and how the use of lasers can enable the clinician to deliver responsible care.
Similar content being viewed by others
Biological considerations
In an otherwise healthy individual, the biological mechanisms that allow healing to take place will always follow the same pathways, irrespective of whether tissue injury is due to a scalpel, thermal, chemical or traumatic cause. Consolidation of wound protection – blood clotting and plasma retention, elimination of bacterial infection and other aspects of classical inflammatory response – is followed by an in-growth of epithelial and endothelial cell types, which then proceeds to maturation of wound healing over time. Any potential for scar tissue formation can be affected by the type of tissue, presence of tissue mediators and growth factors, the cause of the wound, whether healing is by primary or secondary intention and, occasionally, racial type.1,2
In an ideal situation, the post surgical healing will be such as to restore stability, form and function to the tissue. In oral soft tissue surgery, where appropriate, the aesthetics of the tissue will be maintained or, as is often the desired outcome, improved with regard to fixed restorations.
Whenever soft tissue is incised using a scalpel, there will a succession of events that will dictate tissue management:
-
i
Bleeding: most intra-oral soft tissue procedures associated with general dental practice would normally involve the cutting or puncture of small-diameter vessels (arterioles, venules and capillaries)
-
ii
Dressings: the aim of any dressing will be to arrest bleeding and allow clot formation, stabilise the cut margin, stabilise tissue to allow healing and prevent possible disturbance of the incision
-
iii
Contamination: the ingress of bacteria into the incision site, sutures and dressings is inevitable and will act to compromise the inflammatory response. This will often add to any post-operative pain or discomfort
-
iv
Short-term follow-up: removal of sutures and/or dressings
-
v
Long-term: re-organisation of epithelial and endothelial component structure with possible shrinkage.
Assuming correct laser wavelength per tissue site and appropriate power parameters, the healing of oral soft tissue is often termed 'uneventful'. Often, if not always, the need for dressings or sutures is avoided. Irrespective of the wavelength, all soft tissue healing will be by secondary intention, in that it will be impossible to oppose the cut tissue edges to their original alignment. Of note, however, is the phenomenon of lack of post-incisional contamination by bacteria, due to a possible sterility of the cut surface,3 but certainly through the protective layer of coagulum of plasma and blood products – a tenacious film that allows early healing to take place underneath.4 Additionally, studies with longer wavelengths show that there is a lack of fibroblast alignment associated with the incision line and consequent reduced tissue shrinkage through scarring.5 Such findings are often borne out in the clinical setting.
Surgical considerations
The use of a laser, as with more conventional instruments, demands of the clinician basic surgical skills which should remain paramount. Knowledge of the anatomical site, sound diagnostic skills, appreciation of the desired post-surgical outcome and functional needs should be combined with a thorough understanding of the patient's dental and medical history. Where appropriate, the nature of any pathology should be assessed prior to surgical intervention and referral protocols for specialist care should apply, if necessary.
'Loose' soft tissue surgery
Within general dental practice, this would include the removal of fibromata, mucocoele, small haemangiomata, denture granulomata, labial and lingual fraenectomies and treatment of non-erosive lichen planus and mucocytosis.
The aetiology of the lesion should be assessed, together with an understanding of the tissue composition. As with a scalpel, the abnormal tissue, if possible, should be placed under tension to allow accurate cleavage. In most cases, the laser hand-piece tip is held in close approximation to, and just out of contact with, the tissue surface. In this way, the laser energy is allowed to effect the incision and minimise the build-up of debris on the tip, which can distort the laser-tissue interaction.
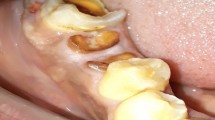
As was seen in previous articles in this series, 'safe' soft tissue cleavage, avoiding the potential of collateral thermal damage, is related to correct wavelength/tissue assessment, minimum laser power to achieve tissue cleavage and thermal relaxation measures to prevent heat build-up. Shorter laser wavelengths (diode, 810, 980 nm; Nd:YAG, 1,064 nm) transverse the epithelium and penetrate two to six millimetres into the tissue, whereas longer wavelengths have minimal penetration. As surgical cutting proceeds, the heat generated seals small blood and lymphatic vessels, reducing or eliminating bleeding and oedema. Denatured proteins within tissue and plasma give rise to a surface zone of a tenacious layer, termed 'coagulum' or 'char', which serves to protect the surgical wound from frictional or bacterial action. Clinically, during 48-72 hours post-surgery, this layer undergoes hydration from saliva, swells and disintegrates and eventually is lost to reveal an early healing bed of new tissue. This sequence of events can be seen in the removal of a lingual fibroma using a Nd:YAG laser. The area of reactive tissue oedema surrounding the ablation site indicates the penetrating conductive heat effects found with shorter wavelength lasers, such as the Nd:YAG (Figs 1,2,3,4,5).
This risk of collateral thermal damage can be minimised by directing the laser beam into the discard tissue (Figs 6,7,8,9).
With longer wavelengths (Er,Cr:YSGG, 2,780 nm; Er:YAG, 2,940 nm; CO2, 10,600 nm), the risk of deep penetration is minimised and surgical incisions can be deemed less potentially damaging. However, in the author's experience, the spot size of most CO2 lasers is larger than the fibre optic-delivered shorter wavelengths and hand-piece tips used with erbium lasers, which renders incisions with CO2 wavelength potentially less accurate. This has little consequence in 'loose' soft tissue surgery, but may have an effect in detailed surgery, where aesthetics are a prime consideration (Figs 10,11,12,13).
Shorter, visible and near-infrared wavelengths are readily absorbed by pigmented tissue. This can be used advantageously in the treatment of small haemangiomata, especially those of possible traumatic origin (Figs 14,15,16,17).
Laser fluence levels and surgical incisions
The objective of correct ('safe') laser energy per surgical site (fluence – energy density – J/cm2) shall be to use the minimum level, commensurate with the desired effect.
Insufficient laser energy levels may not initiate tissue ablation, whereas excessive levels can lead to carbonisation and possible deep collateral thermal damage. Carbon, whether present as a build-up on a fibre tip or tissue surface, absorbs all light wavelengths and quickly over-heats. This becomes a source of secondary thermal energy and acts as a 'branding iron', leading to conductive thermal damage.
For most intra-oral minor surgical procedures, an average laser power (J/s) setting should be in the range of two to four Watts. This is based on personal experience and recommended levels found in manufacturers' user manuals. Whilst this might be easy to comprehend in those continuous-wave (CW) emission lasers (diode 810-980 nm and CO2), where the machine panel display is the average power output, for those free-running (FRP) lasers where energy per pulse and pulse numbers are displayed, the average power is the product of these values (eg 200 mJ per pulse/15 pulses per s = 3.5 W average power). With regard to the latter, increasing the value of pulses will reduce the thermal relaxation potential. This is less of a problem with longer FRP wavelengths, eg Er:YAG and Er,Cr:YSGG, due to their shallow penetration, and does indeed allow better coagulative capabilities for these wavelengths with pigmented tissue.
Intrinsically linked to the average power is the close location of anatomical sites that might be damaged through excessive power values6 (Figs 18 and 19). In this case, the penetration of the near-infrared wavelength beam was sufficient to cause thermal damage to the underlying periosteum and bone, resulting in tissue necrosis and a disfiguring cleft in the overlying attached gingiva.
This disastrous result can be compared with a similar procedure, where the same wavelength is used at lower average power values (2.5 W), the laser beam is kept parallel to and away from the underlying bone and sufficient time intervals employed to allow tissue cooling (Figs 20,21,22,23).
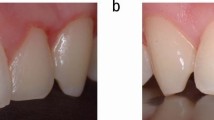
Wherever possible, 'loose' soft tissue incisions can be accomplished more easily if the site is placed under tension. One of the commonest procedures where laser use might be deemed superior to scalpel is the fraenectomy. As with all laser soft tissue surgery, it is essential to appreciate that it is the light energy that is effecting the incision. Using a light 'brush stroke' action, with the delivery tip just out of contact and the tissue under tension, the tissue is seen to 'melt' away. The absence of bleeding significantly reduces post-operative swelling and discomfort and the absence of sutures can minimise the risk of distortion of anatomy. Figures 24,25,26,27 show a case where a revision surgery of a 'failed' scalpel fraenectomy results in a more acceptable result. The Er:YAG laser is readily absorbed by the fibrous scar tissue. Figures 28, 29,30 show a case of a lingual fraenectomy, on a 15 year-old patient, using a diode (810 nm) laser.
Where flat surface pathology requires excision, the minimal penetration of the CO2 laser wavelength can be used to advantage in performing a 'laser peel'. This requires a 'de-focussed' or long non-contact technique, to prevent incisional-type cutting. The 'char' layer produced with this technique is removed with damp gauze, allowing the procedure to be completed to the desired depth. Lesions such as non-erosive lichen planus, where specialist referral and biopsy have endorsed a benign diagnosis, can be treated successfully with minimal distortion of anatomical structures (Figs 31,32,33).
Another clinical situation where gross tissue removal makes haemostasis, bacterial control and dressings difficult, could be the removal of hyperplastic 'denture' granulomata. As Figures 34,35,36,37,38 show, the use of a laser (diode 810 nm in this case) allows tissue excision, management and healing to be accomplished with minimum disruption.
Summary
An overview has been given of the interaction of laser light with 'loose' soft tissue structures in the mouth. The advantages of this modality in the approach to surgery have been given, together with the need to match laser energy levels and absorption potential with surgical technique and appropriate patient management.
References
Bayat A, Arscott G, Ollier W E, McGrouther D A, Ferguson M W . Keloid disease: clinical relevance of single versus multiple site scars. Br J Plast Surg 2005; 58: 28–37.
Funato N, Moriyama K, Baba Y, Kuroda T . Evidence for apoptosis induction in myofibroblasts during palatal mucoperiosteal repair. J Dent Res 1999; 78: 1511–1517.
Kaminer R, Liebow C, Margarone J E 3rd, Zambon J J. Bacteremia following laser and conventional surgery in hamsters. J Oral Maxillofac Surg 1990; 48: 45–48.
Nanami T, Shiba H, Ikeuchi S, Nagai T, Asanami S, Shibata T . Clinical applications and basic studies of laser in dentistry and oral surgery. Keio J Med 1993; 42: 199–201.
Fisher S E, Frame J W, Browne R M, Tranter R M . A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa. Arch Oral Biol 1983; 28: 287–291.
Spencer P, Cobb C M, Wieliczka D M, Glaros A G, Morris P J . Change in temperature of subjacent bone during soft tissue laser ablation. J Periodontol 1998; 69: 1278–1282.
Author information
Authors and Affiliations
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Parker, S. Lasers and soft tissue: 'loose' soft tissue surgery. Br Dent J 202, 185–191 (2007). https://doi.org/10.1038/bdj.2007.128
Published:
Issue Date:
DOI: https://doi.org/10.1038/bdj.2007.128
This article is cited by
-
Histological implications of high-power laser use in the oral soft tissue lesions: a systematic review
Lasers in Medical Science (2023)
-
Laser treatment of pilonidal disease: a systematic review
Lasers in Medical Science (2022)
-
Initial treatment for patients with temporomandibular disorders: pain relief and muscle tone relief by photobiomodulation therapy using carbon dioxide laser
Lasers in Dental Science (2020)
-
Optoacoustic monitoring of cutting efficiency and thermal damage during laser ablation
Lasers in Medical Science (2014)
-
The influence of water/air cooling on collateral tissue damage using a diode laser with an innovative pulse design (micropulsed mode)—an in vitro study
Lasers in Medical Science (2013)