Key Points
-
Review of hypersensitivity reactions to denture base resins.
-
Identification of a little recognised allergen in this rare condition, with systemic effects.
-
Review of investigations for such cases, with recommendations on appropriate investigations and treatment options
Abstract
Hypersensitivity reactions to the commonly used denture base resins are infrequently reported. When they have been reported, most acrylic hypersensitivity reactions have been described as local contact reactions with few reports identifying any significant systemic symptoms.
This paper reports a case where the patient suffered extensive systemic symptoms which were strongly linked to denture wear. A variety of alternative dentures of different resin content were constructed over time with varying reactions. The patient was patch-tested and responded with positive reactions to pure dye samples supplied by manufacturers of the resins. She also failed to react to dentures made in a clear acrylic with no dye components. These factors strongly support the hypothesis that the reactions experienced by this patient to some denture resins was the result of the incorporated colouring agents.
It is therefore suggested that in cases where a hypersensitivity reaction with systemic manifestations to a denture base resin is suspected, questioning with regard to other reactions to colourants and patch testing for dyestuffs should be considered in addition to the use of a resin with no colouring agents in construction of replacement prostheses.
Similar content being viewed by others
Main
While hypersensitivity reactions to the commonly used denture base resins, particularly polymethylmethacrylate, are infrequently reported,1 a variety of factors may lead to similar symptoms. Candida associated denture-induced stomatitis,2 burning mouth syndrome3 and simple denture-induced physical trauma4 are all conditions which can be mistaken for an acrylic hypersensitivity reaction.
When they have been reported, most hypersensitivity reactions to acrylic have been described as local contact hypersensitivity reactions with few reports identifying any significant systemic symptoms. The present paper reports a case where the patient suffered extensive systemic symptoms which can be clearly linked to denture wear and which are likely have been a reaction to an element of the resin which previously has been reported infrequently5 as a potential allergen.
Case report
This paper reports the case of a female patient who first attended the Department of Prosthodontics at Glasgow Dental Hospital and School NHS Trust in 1984, when she was 70 years old. Previously the patient had successfully worn complete upper and partial lower dentures for approximately 50 years. When originally referred, she reported a one-month history of a gradually increasing rash affecting her face and neck, with associated swelling and difficulty breathing. At that time it was thought that her symptoms had arisen following a lower dental clearance in December 1983, when an area of new autopolymerising acrylic had been used in the immediate addition of six lower anterior teeth to the existing 14 year old acrylic partial lower denture. The previous complete upper denture had a metal palate and had also been relined at this stage. She did however report an approximately 18 month history of an intermittent rash affecting the skin of her face and neck while wearing the most recent prostheses, although she had worn them apparently successfully for nearly 14 years.
She was prescribed antihistamines at this time, although the patient reported that following onset of the above symptoms, she stopped wearing those dentures and the symptoms resolved. She was provided with replacement acrylic prostheses in December 1984. She encountered some symptoms following this, complaining of feeling 'drunk' and of oral discomfort, facial swelling and an itch.
She was referred to the Contact Dermatitis Investigation Unit at Glasgow Royal Infirmary at this point and was shown to react to scrapings of the denture resin used. Although no definite hypersensitivity to acrylic per se appears to have been identified at this time, it was decided to provide her with vulcanite dentures, with which she appeared to cope very well for a number of years. When these became loose in 1988, replacement dentures were constructed in nylon (vulcanite no longer being readily available), and she appeared again to show no significant reaction to this material. These dentures were replaced in 1993 with new nylon dentures and the patient generally coped well with these, with no evidence of an adverse reaction. However, as she had some persistent minor local symptoms related to base adaptation, the lower denture was remade in nylon using a copy technique in December 1994, at which point the patient was 80-years-old, following which she reported significant systemic symptoms, including a general malaise, oral discomfort, throat swelling, blurred vision and dizziness. These symptoms apparently appeared within about 90 minutes of the dentures being placed. In view of her previous history in relation to denture wear, the patient discontinued wearing her dentures and her symptoms gradually receded.
In an attempt to establish a link between the symptoms and denture wear, she was asked to return to the Dental Hospital having worn the dentures for a few hours. On arrival, she reported that she had felt well on rising, but since inserting the dentures had felt increasingly unwell. She exhibited a marked tremor, felt unsteady on her feet, was pallid and her pulse rate was elevated. Her mouth was dry and uncomfortable. No obvious oral lesions were noted however, this being the case throughout the period reported, other than occasional areas which could be attributed to denture-induced trauma.
She was referred to the Department of Oral Medicine, where a routine blood screen showed no significant abnormalities, although a random blood glucose was slightly elevated beyond the normal range. Following this episode, the patient removed her dentures and her symptoms resolved within a few days.
The patient was re-referred to the Contact Dermatitis Investigation Unit at Glasgow Royal Infirmary. The patient had previously reported an intolerance of anything coloured red, such that even the sight of a red article could lead to physical symptoms including vomiting. She has also reported problems with red coloured food and red tablets (Brufen) all of which may contain erythrosine dye. This raised the question as to whether she might be having a reaction to the dyestuffs in the denture resins, as colour was a common factor in the denture materials used, which were otherwise of a very different composition. The patient also reported a reaction to the use of Poli-grip Ultra denture adhesive (Stafford Miller), which also contains red colouring agents. Interestingly, the patient also reported that a nephew living in New Zealand and whom she had not seen for many years had also recently reported an intolerance of anything red.
Samples of various dye materials used in the acrylic denture resins (Trevalon: De Trey) used in the department, along with samples of the dyes in Poli-grip Ultra were obtained from the manufacturers. It proved impossible to obtain samples or even information on dyestuffs used in the nylon material as the German manufacturer of the particular material used (Flexiplast) was not prepared to provide these on the grounds of commercial confidentiality. The dye materials investigated are listed in Table 1.
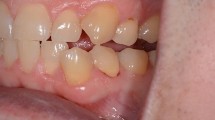
While awaiting communication from the suppliers, the patient expressed concern at not having any dentures which she could successfully wear and it was therefore decided to provide replacement complete dentures made in Trevalon C (De Trey), a clear heat cure acrylic, and to use standard acrylic teeth (Senator: Wright Dental). These were provided in November 1995 (fig. 1) and to date she has had no systemic symptoms, only complaining of occasional local problems related to mechanical denture-induced trauma.
Following receipt of the dyestuff samples, the patient underwent patch testing with the standard European battery and to an extended battery of dental materials including the dyestuff materials supplied. In the period immediately following these investigations, the patient reported significant systemic symptoms as previously described, with nausea, dizziness, swelling and general malaise. These symptoms persisted until the test materials were removed.
Discussion
A number of constituents of the resin, in both its unpolymerised and polymerised state, have been implicated as putative allergens giving rise to reactions similar to that experienced by the patient. These include the unpolymerised acrylic monomer methylmethacrylate,1,6 the initiator benzoylperoxide, hydroquinone,7 formaldehyde and plasticising agents.1 The response to the materials has usually been described as a locally irritant reaction, with only occasional references to systemic effects such as 'asthma, soreness and itching',8 or 'swollen ankles and dizziness'.5
Methylmethacrylate has been recognised, in both denture base resins and in temporary crown materials,1,9,10 as giving reactions which have been described as 'a tingling sensation and swelling' or 'burning and itching sensations'.
Some studies have attempted to assess the extent to which components of the resin leach from the material in the oral environment, using assay techniques to identify the materials in saliva,9,11 and also looking at the cytotoxic effects of eluates of denture base resin on tissue culture.12 However, in common with other reports, these papers have not identified individual elements of the resin as being responsible for the reaction.
There are 5 recognised types of hypersensitivity reaction.13 The case presented exhibited signs and symptoms consistent with a Type III (Immune complex mediated) hypersensitivity. Such reactions arise as a result of formation of insoluble antibody/antigen complexes which may lodge at various sites within the body.
The effects of formation of these complexes are dependent upon both the absolute and relative amounts of antigen and antibody in the complexes. The absolute amount will govern the intensity of the reaction while the relative proportions determine the type of complex formed and its distribution in the body.
The reaction may be local, as in the Arthus reaction, producing a localised erythema and oedema of a few hours duration as a result of mainly intra-vascular changes close to the site of allergen presentation.
Systemic effects may also arise from the presence of circulating complexes. This includes so-called 'serum-sickness' where injection of foreign serum for therapeutic purposes (eg horse antidiphtheria) may lead to systemic symptoms of pyrexia, lymphadenopathy, generalised urticarial rash and joint swelling at approximately one week after administration.
The symptoms of swelling, rashes and general malaise of which the patient complained within variable but usually short periods following exposure to the 'allergen' — her dentures — suggest that she was experiencing a Type III hypersensitivity reaction.
Conclusion
The patient has during her lifetime worn complete dentures made of a range of resins with varying success and degree of reaction to the different materials. It is unfortunate that further data on the constituents of the nylon denture base resin are not available. However, the reaction achieved to the pure dye samples and the lack of reaction to the clear acrylic dentures is strongly suggestive of the fact that the reactions experienced by this patient to some denture resins is the result of the incorporated colouring agents. Only one other report in the dental literature appears to have recognised the possibility of dyestuffs being involved in the patient's reaction to the prostheses.5 In common with the present case, the patient experienced systemic symptoms including swelling and dizziness and the symptoms resolved following provision of dentures constructed in a clear resin.
It has been suggested14 that colourants used in the pharmaceutical industry may be responsible for some of the large numbers of drug reactions seen and usually attributed to the drugs themselves. The author reported an incidence of a patient taking Naproxen who, when changed to a different formulation of the same drug, with a different coloured coating, developed a severe allergic skin reaction.
It is therefore suggested that in cases where a hypersensitivity reaction with systemic manifestations to the denture base resin is suspected, the use of a resin with no colouring agents incorporated should be considered to exclude the possibility of hypersensitivity to dyestuffs. In addition, referral to a consultant dermatologist to allow further investigation and patch testing for such a reaction should be considered.
References
Devlin H, Watts D C . Acrylic 'Allergy'. Br. Dent. J. 1984: 157; 272–75.
Weaver R E, Goebel W M . Reactions to acrylic resin dental prostheses. J. Prosthet. Dent. 1980: 43; 138–142.
Ali A, Bates J F, Reynolds A J, Walker D M . The burning mouth syndrome related to the wearing of acrylic dentures: an investigation. Br. Dent. J. 1986: 161; 444–447.
Nyquist G . Study of denture Sore mouth. Acta Odontol. Scand. 1952: 10; Supplement 9, 13–17.
Sim J . Allergic reaction to Denture Base Material. J. Can. Dent. Assoc. 1958: 24; 292–294.
Strain J C . Reactions associated with acrylic denture base resins. J. Prosthet. Dent. 1967: 18; 465–468.
Torres V, Mano-Azul A C, Correia T, Soares A P . Allergic contact cheilitis and stomatitis from hydroquinone in an acrylic dental prosthesis. Cont. Derm. 1993: 29; 102–103.
Stungis T E, Fink J N . Hypersensitivity to acrylic resin. J. Prosthet. Dent. 1969; 22: 425–428.
Guinta J L, Grauer I, Zablotsky N . Allergic contact stomatitis caused by acrylic resin. J. Prosthet. Dent. 1979: 42; 188–190.
Nealey E T, del Rio C E . Stomatitis venenata: Reaction of a patient to acrylic resin. J. Prosthet. Dent. 1969: 21; 480–484.
Koda T, Tsuchiya H, Yamauchi M, Ohtani S, Takagi N, Kawano J . Leachability of denture base acrylic resins in artificial saliva. Dent. Mater. 1990: 6; 13–16.
Schuster G S, Lefebvre C A, Dirksen T R, Knoernschild K L, Caughman G B . Relationships between denture base resin cytotoxicity and cell lipid metabolism. Int. J. Prosthodont. 1995: 6; 580–586.
Roitt I. Essential Immunology, Ninth Edition, Blackwell Science, Oxford, England, 1997; chapter 16, 328–352.
Bell T . Colourants and drug reactions (letter). Lancet. 1991: 338; 55–6.
Author information
Authors and Affiliations
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Barclay, S., Forsyth, A., Felix, D. et al. Hypersensitivity to denture materials. Br Dent J 187, 350–352 (1999). https://doi.org/10.1038/sj.bdj.4800278
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.4800278