Key Points
-
Seriously discoloured teeth amount to a physical handicap and cannot be dismissed as being merely a 'cosmetic' matter.
-
Ten percent peroxide (Opalescence gel) held within a customised tray effectively and safely bleaches teeth by penetration through enamel and into dentine. This is not a superficial or cosmetic action.
-
Ten percent cabamide peroxide is completely safe from the viewpoint of general toxicity, local toxicity, risk of mutation and risk of carcinogenesis. It does not damage the hardness of teeth nor dental pulp. There are no adverse soft tissue effects.
-
The legal position as to its regulation as a medical device or as a cosmetic device remains in dispute in the UK.
Abstract
Fears that the dentist-supervised use of a product that contains carbamide peroxide and that emits hydrogen peroxide may not be safe from the viewpoints of toxicity and cancer risk were engendered by unrealistic animal tests. These fears prompted the UK Government Departments of Trade and Industry (DTI) and of Health (DOH) to try to prohibit the marketing of Opalescence (manufactured by Ultradent Inc.). Faced with the fact that Opalescence had already been awarded a CE mark under the EC Medical Devices Directive, the DTI and DOH attempted to bring about its prohibition by reclassifying Opalescence as falling under the EC Cosmetics Directive, according to which the marketing of products containing more than 0.1% hydrogen peroxide is not permitted.
Similar content being viewed by others
Main
The rationality and legality of this manoeuvre were tested in the UK Courts in 1998 and the classification of Opalescence as falling under the Medical Devices Directive was upheld. The DTI and DOH appealed against the decision. In 1999 the original judgement was overturned by the Court of Appeal. The matter has now been referred to the House of Lords in the UK.
In this short review we discuss why Opalescence should be classified as a medical device, why exposure to the small amounts of hydrogen peroxide which it emits poses no concern on the grounds of potential general toxicity or carcinogenicity, the evidence for its efficacy and the evidence of its freedom from adverse effects on teeth and the soft tissues of the mouth.
Introduction
Noticeable discoloration of teeth can be a physical handicap that impacts on a person's self-image, self-confidence, physical attractiveness and employability. It can not therefore be dismissed as simply of cosmetic importance.
Dentists have sought to improve the appearance of discoloured teeth by a variety of methods for centuries. Discoloured teeth have been bleached by dentists for at least 100 years.1 Many of these methods involved the use of hydrogen peroxide in varying concentrations using a variety of techniques.
Haywood and Heymann2 described a method of bleaching vital teeth which involved the application of carbamide peroxide in the form of a viscous gel held within a customised mouthguard (sometimes referred to as a tray). The gel was 10% carbamide peroxide which released hydrogen peroxide. The carbamide peroxide gel was thickened by the addition of carbopol. The gel, held within the mouthguard, was applied to the discoloured teeth at night while the patient slept. This was termed 'nightguard vital bleaching'. This method was extensively tested and proved to be very successful without producing significant side effects.3
Opalescence is a product manufactured by Ultradent Inc. based on the method developed by Haywood and Heymann.2 It is a device with several components. The device and its use, which are described in Annex A, were introduced into the UK market by Optident Ltd. However, in 1993 the DTI advised that the product was (1) regulated under the EC Cosmetics Directive and (2) not permitted for dental bleaching because it contained more than 0.1% hydrogen peroxide (equivalent), a concentration in excess of the limitations of the Cosmetics Directive.
In 1995 Opalescence was granted a CE mark as a medical device under the Medical Devices Directive (MDD) – a new EC regulatory regime first implemented at this time. The consequences of a product getting a CE mark is that it may be marketed in all countries throughout the EC without any further authorisation. This right must be respected by member states.
The UK Government agencies, ie the Medical Devices Agency (MDA), the DTI, and the DOH, took the view that bleaching agents intended to be placed in contact with the teeth are cosmetic products within the meaning of the Cosmetics Products (Safety) (Amendments) Regulations 1993 through which the EC Cosmetics Directive was implemented. For this reason, products intended for bleaching teeth were prohibited from sale in the UK if they contained, or released, more than 0.1% hydrogen peroxide.
It seems that the DTI, the MDA and DOH had been advised that the use of products containing more than 0.1% hydrogen peroxide was dangerous because of the mutagenic and carcinogenic potential of hydrogen peroxide. In spite of numerous requests by the BDA, dentists and others to the DTI, the MDA and the DOH, no evidence to substantiate these claims was offered. Furthermore, the DTI advised that any dentist using such a product would be liable to a fine of £5000, a prison sentence of 6 months or both. Needless to say, this effectively dissuaded most dentists in the UK from using such products.
In due course, the manufacturers, Ultradent Inc., and the UK distributors, Optident Ltd., sued the DTI and the DOH in the High Court, claiming that they had placed illegal obstacles to the marketing of Opalescence in the UK. The legal position with regard to the use of Opalescence was tested in a High Court Case which ended in August 1998. The outcome of this case was that:
-
Opalescence was and is a medical device and not as a cosmetic product;
-
the CE mark as a medical device was and is appropriate; and
-
the DTI and DOH had placed obstacles to its being placed on the market in the UK and this they had not been entitled to do.
The DTI and DOH appealed against the decision. In 1999 the original judgement was overturned by the Court of Appeal. The matter has now been referred to the House of Lords. Dental practitioners may still feel a need to be reassured about the safety of carbamide peroxide-based products. The following sections address these concerns.
It is noteworthy that, despite the fact that the FDA and other bodies in the USA apply very stringent licensing regulations, especially in the matter of safety, the American Dental Association's seal of approval was granted to Opalescence.
Questions concerning general toxicity, possible mutagenicity and possible cancer risk
Is there any risk of local or systemic toxicity to patients from the use of carbamide peroxide by dentists?
Unless the dose is taken into account, there is nothing to which humans are exposed that can be said to be entirely free of the risk of toxicity. Thus, there is an expression that is well known to toxicologists, 'The dose makes the poison'.4
Carbamide peroxide is formed from urea and hydrogen peroxide. Urea is a normal body constituent and in the doses of carbamide peroxide administered by dentists in the course of nightguard vital bleaching involving the use of Opalescence, the urea moiety of the molecule is of no toxicological consequence.
After the application of carbamide peroxide to teeth, hydrogen peroxide is slowly emitted and it is this that is absorbed into teeth and results in their being bleached. The question arises therefore, is there any risk of local or general toxicity from the hydrogen peroxide that is emitted from the carbamide peroxide?
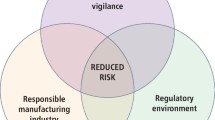
The toxicology of hydrogen peroxide (H2O2) was thoroughly reviewed in 1993 by the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC5) and later by Li.6 The main points made in the ECETOC review are shown in figure 1.
As pointed out below, the process of the conversion of normal foodstuffs to the energy required for the growth, movement and survival of animals, including humans, involves the production of oxygen free-radicals, including H2O2. Consequently, H2O2 can be found in all body cells as an endogenous metabolite. Boveris7 calculated that the human liver – the principal site of metabolism – produces 270 mg of H2O2 per hour. An important reason why there are no bad consequences of this generation of H2O2 is the effective way in which H2O2 is decomposed by enzymes, particularly catalase and various peroxidases. All body cells contain these enzymes, but the highest levels are found in the duodenum, liver, spleen, kidney, blood and mucous membranes. In the blood, most of the catalase is found in red blood cells and within just a few minutes the erythrocytes can degrade gram quantities of H2O2.
Is there a risk of mutagenesis from exposure to hydrogen peroxide?
No such risk is supported by the best available scientific evidence. A balanced and thorough review of the scientific evidence indicates that clinically useful concentrations and applications of hydrogen peroxide do not entail a risk of mutagenesis in humans. Original questions about the role of hydrogen peroxide in mutagenesis were raised on the basis of certain in vitro testing results, which have not been reproduced under in vivo testing conditions.9,10
Is there any risk of carcinogenesis as a consequence of the exposure of patients to carbamide peroxide during dentistry?
Major advances in knowledge concerning mechanisms involved in carcinogenesis have occurred during the last 10–15 years.11 Ames12 pointed out that the human diet contains a wide variety of natural mutagens and carcinogens, and also many ingredients which, when metabolized, generate potentially mutagenic oxygen radicals. Endogenous mutagens so formed cause massive DNA damage (ie by the formation of oxidative and other adducts) that can be converted to stable mutations during cell division.13 These investigators estimated that, on average, the DNA of each cell in the human body suffers 10,000 hits per day from endogenous oxidants. Almost all of this damage is quickly and effectively repaired. However, there is a brief stage in the process of cell-division when the normally double-stranded DNA is only single stranded. During this stage DNA-repair is impeded. It follows that agents (eg irritants, hormones) that stimulate cell division (ie mitosis) increase the risk that DNA damage will be transferred to daughter cells. Ames and Gold13 coined the expression “Mitosis increases mutagenesis” to describe this phenomenon. In the light of this it becomes easy to see how non-mutagenic agents that stimulate cell division might increase the risk of cancer development.
Animal studies conducted in Japan by Ito et al.14,15,16 described the occurrence of benign and malignant tumours of the duodenum in mice exposed continuously to 0.1% or 0.4% H2O2 in their drinking water for up to 100 weeks. In the IARC monograph8 it was noted that the pathology of the tumours that were said to have arisen in the case of the 1984 paper 'was not well documented'. This was probably also true for the earlier two papers, although this is not clear from the contents of the 1985 IARC monograph. In their 1984 paper Ito et al.,16 reported that the level of catalase in the duodenum in mice of four strains ranged from 76 to 1000 times lower than that in the duodenum of one strain of rat. As catalase is a potent destroyer of H2O2, it is likely that duodenal deficiency of catalase could increase the risk of damage, and possibly also of neoplasia, in such strains of mice. In this regard, Dawson et al.17 found that catalase levels in the duodenum of humans were 2–27 times higher than in any of the mouse strains that they studied. Clearly, the reported production of corrosive changes and duodenal tumours in mice exposed continuously to H2O2 in the drinking water is not an appropriate model for assessing the possible risks of duodenal irritation or tumorigenesis in man.
Ito et al.18 exposed rats on 6 days/week for 12 weeks by gavage to 168.7 mg/kg bodyweight H2O2 (the equivalent of a human swallowing over 10 g of H2O2). They saw no histopathological abnormalities in any tissue. Later, Ishikawa and Takayama19 studied the effects on the duodenum of Fischer 344 rats that were exposed to 0.3% or 0.6% H2O2 in the drinking water for 26 weeks and then observed for a further 52 weeks. They observed no duodenal tumours in this study. In contrast, in the light of the results of another study in rats, Dahl and Becher20 concluded that hydrogen peroxide in dental whiteners might be hazardous for humans. These investigators exposed rats to doses of either 15 or 50 mg/kg body weight carbamide peroxide or to 150 or 500 mg/kg Opalescence by stomach tube, after overnight starvation. In rats so treated they saw erosion of the gastric mucosa of dose-dependent severity after 1 hour and after 24 hours. However, their findings are difficult to interpret in relation to the use of carbamide peroxide for tooth whitening. Firstly, the method of administration served to maximise exposure of the gastric mucosa by avoiding dilution by saliva or food. Secondly, as the investigators themselves commented, the irritant effects they saw were only very transient.
Common sense dictates that it is the concentration of H2O2 that comes into contact with particular tissues and not the total dose on a mg/kg body weight basis that determines manifestations of local toxicity.
In our opinion the experiment of Dahl and Becher20 is not relevant in relation to the safety evaluation of the overnight use of Opalescence by dentists. It is well known that high concentrations of H2O2 are corrosive to any tissue that comes into contact with them, and this is all that Dahl and Becher showed in their study in rats. The use of Opalescence does not entail the exposure of tissues other than the teeth to toxicologically significant concentrations of H2O2.
Questions of safety of particular relevance to dentists
Does exposure to carbamide peroxide increase resorption?
Resorption frequently occurs as a result of trauma to teeth. The severity of resorption is related inter alia to the type of injury the tooth has sustained, the force involved and whether the tooth is dislodged from its socket. Thus, resorption can and does occur without any exposure to internal bleaching involving H2O2.
Invasive cervical resorption is seen very occasionally in bleached root-filled teeth, and has been attributed to a combination of trauma to the tooth followed by the use of heat and very high concentrations of hydrogen peroxide, eg 30%.21 Heithersay et al.22 studied the radiographs and records of 204 teeth in 158 patients, whose bleaching treatment had been carried out in a specialist endodontic practice over periods of 1–19 years. In 78% there was a history of traumatic injury. All teeth had been treated with a combination of thermo-catalytic (heat-activated 30% H2O2) and 'walking bleach' procedures. It was found that a total of four teeth from the sample group (2%) had developed invasive cervical resorption during the review period. All of these teeth had a history of traumatic injury and the level of root filling was at the cemento-enamel junction.
Resorption appears to be more a function of the application of heat and of high concentrations of H2O2. There appears to be no evidence in the scientific literature implicating the use of H2O2 in resorption when heat is not used or when only low concentrations of H2O2 (ie 3%) are used. In other words, it is only when a combination of high concentration of H2O2 and heat are used on teeth with a history of trauma that resorption very occasionally results. Incidentally, there are no reported cases of resorption with the use of Opalescence used for either internal or external bleaching.
Does increased tooth sensitivity associated with the use of carbamide peroxide constitute a serious problem?
Schulte and Morrisette23 reported a clinical study involving home bleaching with 10% carbamide peroxide. There were 28 people in the study. Four of these discontinued the procedure because of thermal sensitivity. For the remaining people, there was no difference between the pulpal readings recorded before the use of the gel and those at any point during the study. During a 3-week trial by Sterrett et al.,24 it was reported that mild transient sensitivity was common to all participants. The consensus view from these and other trials was that this mild sensitivity ceased on stopping treatment. Further details are available from the papers presented at a state-of-the-art symposium on non-restorative treatment of discoloured teeth in September 1996 at the School of Dentistry, North Carolina, Chapel Hill. These papers were subsequently published in the Journal of the American Dental Association Supplement.3
From these recent studies and from comparisons of bleaching by traditional methods with bleaching using Opalescence, it is clear that increased sensitivity is mainly associated with the use of heat and high concentrations of H2O2.25,26,27 Neither heat nor high concentrations of H2O2 are involved in the use of Opalescence.
Does bleaching adversely affect the hardness of teeth?
Lewinstein and Hirschfeld28 claimed that hydrogen peroxide might reduce the hardness of enamel and dentine. However, their study was flawed because they used H2O2 at pH3. This would, in effect, acid etch the enamel surface of teeth. In this context Opalescence has a pH of 6.5 and would not give rise to acid etching. In any case, the apparent small decreases in microhardness were only on the borderline of statistical significance. If, indeed, there are any changes in tooth hardness after bleaching they are certainly likely to be far less than those resulting from the removal of the enamel before the application of veneers or changes associated with micro abrasion.29,30,31
Can carbamide peroxide be safely used for the internal bleaching of teeth?
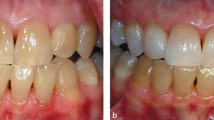
Prior to the1998 court hearing referred to in the introduction, the DTI and the DOH were seemingly labouring under the false belief that the bleaching of teeth by Opalescence resulted from an effect solely on the surface of teeth. In fact this is simply not true as shown by the response of tetracycline-stained teeth to Opalescence treatment. The yellow or brown/grey staining attributable to tetracycline is located deep in the dentine that is to say, at some distance from the enamal surface of the tooth. Hydrogen peroxide emitted from carbamide peroxide applied to the surface of teeth has to penetrate the enamel and dentine for the bleaching of tetracycline-stained teeth to be effective.
Thus, even when carbamide peroxide is simply applied to the exterior of a discoloured tooth, the benefit it achieves may be wholly or partly brought about by bleaching stains or other compounds within the substance of the tooth as distinct from mere surface discoloration.
However, it is also possible in the case of a non-vital tooth, for the dentist to drill through the wall of the tooth into the tooth pulp chamber and introduce a bleaching agent into the centre of the tooth. This is the usual meaning of the term 'internal bleaching'.22
Another variation of bleaching treatment has been described by Settembrini et al.32 This is known as 'inside/ outside non-vital tooth bleaching' and involves the application of the bleaching agent both internally and externally at the same time. Very successful results have been claimed for this technique but problems of microleakage need to be weighed against improvement in appearance.
In any event, it is clear that carbamide peroxide can be safely used for the internal bleaching of teeth provided that care is taken to avoid the introduction of bacteria into the root canal.
Does damage to the soft tissues of the mouth occur during overnight bleaching with Opalescence?
The American Dental Association's Guidelines for the acceptance of peroxide products published in 199433 require inter alia an evaluation of the effects of treatment with Opalescence on the soft tissues of the mouth, including the gingivae, tongue, lips and palate. Relevant studies are by Sterrett et al.,24 Curtis et al.34 and Russell et al.35 In none of these has soft tissue damage of concern been observed. Furthermore, where transient damage to gingivae was seen, it appeared to be related to poorly fitting trays rather than to the carbamide peroxide gel which they contained.
Is tooth-bleaching with Opalescence effective and long- lasting?
The JADA guidelines33, which are very strict, require companies to show both the safety-in-use of products and their efficacy.
In relation to efficacy the data required included:
-
two double blind trials, involving the comparison of the test material with a non-active control material;
-
the assessment of the effects of treatment over periods of from 2 to 6 weeks;
-
the measurement of tooth colour at the start and at the end of treatment using two different systems of colour measurement, and
-
3–6 month colour duration measurements to assess whether colour improvement is maintained.
After extensive evaluation, three products, including Opalescence, were given the American Dental Association's (ADA) Seal of Approval in 1997 (ref 3) This shows that the ADA was satisfied that efficacy had been convincingly demonstrated.
Other reviews of the safety and efficacy of tooth-bleaching by carbamide peroxide have been provided by Haywood et al.36 and by Goldstein.37
Conclusions
Noticeable discoloration of teeth can amount to a physical handicap which impacts on a person's self-image, self-confidence, attractiveness and employability. It should not therefore be dismissed as a matter of no more than cosmetic importance.
Hydrogen peroxide liberated from carbamide peroxide is effective in bleaching teeth because it penetrates through the enamel and into the dentine. Thus bleaching is not achieved solely by a surface effect.
In our opinion Opalescence, when used under the supervision of dentists, is completely safe from the viewpoint of general toxicity, risk of mutation and risk of carcinogenesis.
The efficacy of Opalescence in the bleaching of teeth is established.
When properly used, there are no more than minimal adverse effects on the soft tissues of the mouth and these are very transitory.
Mr Justice Laws in the 1998 High Court action referred to above stated: '...it is common ground that the UK Authorities have not sought to invoke Article 8 and 18 of the Medical Devices Directive (ie the clauses concerned with product safety)'.
The outcome of the appeal to the House of Lords is awaited and dentists have been advised against using hydrogen peroxide in any concentrations greater than 0.1%. In other words, all forms of bleaching regardless of the methods employed i.e. internal or external have been proscribed. This will place many dentists in a very difficult ethical and moral position as to the appropriate treatment for their patients with discoloured teeth. This article addresses the safety and efficacy issues of dental bleeching
Both the authors of this paper served as expert witnesses in the High Court case referred to in the introduction
References
Fasanaro T S . Bleaching teeth:history, chemicals and methods used for common tooth discoloration. J Esthet Dent 1992; 4: 71–78.
Haywood V B, Heymann H O . Nightguard vital bleaching. Quintess Int 1989; 20: 173–176.
Reports from an International Symposium on Non-Restorative Treatment of Discoloured Teet. JADA 1997; 128(Suppl): 1S–64S.
Ottoboni M A . The dose makes the poison. Berkeley: Vincent, 1989.
ECETOC. Joint Assessment of Commodity Chemicals No. 22: Hydrogen peroxide [CAS No.7722–84–1). European Centre for Ecotoxicology and Toxicology of Chemicals 1993.
Li Y . Biological properties of peroxide-containing tooth whitener. Fd Chem Toxicol 1996; 34: 887–904.
Boveris A, Oshino N, Chance B . The cellular production of hydrogen peroxide. Biochem J 1972; 128: 617–630.
IARC. Hydrogen peroxide: evaluation of the carcinogenic risk of chemicals to human. 1985; 36: 285–314.
Kawachi T, Yahagi T, Kada T, et al. Co-operative programme on short-term assays for carcinogenicity in Japan. In Molecular and cellular aspects of carcinogen screening tests (ed. Montesano R, Bartsch H & Tomatis L). IARC, 1980.
Keck M, Stehlik G, Binder W . Mutagenitaetsuntersuchunger von Wasserstoffperoxid-bzw Wasserstoffperoxid-Katalase behandelter Milc. Oesterreichische Milch Wirtschaft 1980; 2: 7–14.
Roe F J C . A brief history of the use of laboratory animals for the prediction of carcinogenic risk for man with a note on needs for the future. Exp Toxic Pathol 1998; 50: (in press).
Ames B N . Dietary carcinogens and anticarcinogens: oxygen radicals and degenerative disease. Science 1985; 221: 1256–1264.
Ames B N, Gold L S . Too many rodent carcinogens: Mitogenesis increases mutagenesi. Science 1990; 249: 970–971.
Ito A, Wanatabe H, Naito M, Naito Y . Induction of duodenal tumors in mice by oral administration of hydrogen peroxide. Gann 1981; 72: 174–175.
Ito A, Naito M, Naito Y, Wanatabe H . Induction and characterization of gastro intestinal lesions in mice given continuous oral administration of hydrogen peroxide. Gann 1992; 73: 315–322.
Ito A, Wanatabe H, Naito M, Naito Y, Kawashima K . Correlation between induction of duodenal tumor by hydrogen peroxide and catalase activity in mice. Gann 1984; 75: 17–21.
Dawson J, Bryant M G, Bloom S R, Peters T J . Characterization of gut hormone storage granules from normal human jejunum using metrizamide density gradient. Regulatory Peptides 1981; 2: 305–315.
Ito R, Kawamura H, Chang H et al. Oral safety of hydrogen peroxide, acute and chronic toxicitie. J Medical Soc 1976; 23: 531–537.
Ishikawa T, Takayama S . Cited in Information Bulletin on the Survey of Chemicals Being Tested for Carcinogenicity 1984; 11: 86–95.
Dahl J-E, Becher R . Acute toxicity of carbamide peroxide and a commercially available tooth-bleaching agent in rat. J Dent Res 1995; 74: 710–714.
Friedman S . Internal bleaching: long-term outcomes and complication. JADA 1997; 128(suppl): 51S–55S.
Heithersay G S, Dahlstrom S W, Marrin P D . Incidence of invasive cervical resorption in bleached root filled teeth. Aus Dent J 1994; 39: 82–87.
Schulte J R, Morrisette D B et al. The effects of bleaching application time on the dental pulp. JADA 1994; 125: 1330–1335.
Sterrett J, Price R B, Bankey T . Effects of home bleaching on the tissues of the oral cavity. J Can Dent Assoc 1995; 61: 412–420.
Cohen S C, Parkins F M . Bleaching tetracycline-stained vital teeth. Oral Surg 1970; 29: 465–471.
Cohen S C, Chase C . Human pulpal response to bleaching procedures on vital teet. J Endod 1979; 5: 134–138.
Nathanson D, Parra C . Bleaching vital teeth: a review of clinical study. Compend Cont Educ Dent 1987; 8: 490–497.
Lewinstein I, Hirschfield C et al. The effect of hydrogen peroxide and sodium perborate on the microhardness with human enamel and dentine. J Endod 1994; 20: 61–63.
Haywood V B . History, safety and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintess Int 1992; 23: 471–488.
Haywood V B . Nightguard vital bleaching: current concepts and researc. JADA 1997; 128 (Suppl): 19S–25S.
Shannon H, Spencer P, Gross K, Tira D . Characterisation of enamel exposed to 10% carbamide peroxide bleaching agent. Quintess Int 1993; 24: 39–44.
Settembrini, Gultz J, Kaim J, Scherer W . A technique for bleaching non vital teeth: inside/outside bleaching. JADA 1997; 128: 1283–1284.
Guidelines for the acceptance of peroxide containing oral hygiene. JADA 1994; 125: 1140–1142.
Curtis J W, Dickinson G L, Doney M C et al. Assessing the effects of 10% carbamide peroxide on oral soft tissue. JADA 1996; 127: 1218–1223.
Russell C M, Dickinson G L, Doney M C et al. Dentist-supervised home bleaching with carbamide peroxide gel: a six month study. J Esth Dent 1996; 8: 177–182.
Haywood V B, Leonard R H, Neilson C F, Brunson W D . Effectiveness, side effects and long term status of nightguard vital bleaching. JADA 1994; 125: 1219–1226.
Goldstein R E . In-office bleaching: where we came from, where are we to-day?. JADA 1997; 128 (Suppl); 11S–15S.
Author information
Authors and Affiliations
Annex A Opalescence: the method of using it and the role of the dentist
Annex A Opalescence: the method of using it and the role of the dentist
Opalescence is a device consisting of:
-
1
The material to make a customised tray;
-
2
The block-out material;
-
3
Syringes containing the bleaching gel;
-
4
A container to hold the tray when it is not in use.
The tray material is designed using a model made from an impression. In the UK only a dentist can legally take impressions of patients' teeth.
The light cured 'block out' resin is supplied for use in the fabrication of the tray in order to produce a reservoir for the bleach, as prescribed by the dentist. This serves to limit the volume of bleach to be placed in contact with the area(s) of the teeth to be bleached.
The use of Opalescence involves the following:
-
1
Diagnosis of appropriateness of using a bleaching technique;
-
2
Impression of the patient's teeth taken by a dentist;
-
3
Fabrication of the model of the patient's teeth;
-
4
Use of the block-out material from Opalescence to produce a reservoir;
-
5
Fabrication of the individual tray;
-
6
Dispensing of the tray and appropriate number of syringes of gel with instructions;
-
7
Follow up and assessment of change.
The dentist instructs the patient in its application before dispensing the tray and provides a specific number of syringes of gel for the patient's use. This is usually four doses plus another four doses if needed. In difficult cases, such as Tetracycline discoloration, up to 16 doses may be dispensed under supervision and in the light of a critical review of progress of the colour alteration.
The carbamide peroxide is contained in a sustained release, high-viscosity, sticky gel supplied in measured doses of 1.2 ml (1.2 g) to fill a specially designed dental tray in the areas where it is desired to bleach a row of usually 4–6 teeth. Some of this (up to half of it) is removed after the tray has been placed in the mouth, leaving about 0.6–0.9 g within the tray in the mouth. The filled tray is applied to the teeth, and excess gel is removed. The tray is then left in place for 6–8 hours.
When the tray is removed from the mouth, approximately 0.5 g of the gel remains in the mouth, mostly adhering to the teeth. The patient then brushes his or her teeth and rinses his or her mouth, expectorating (spitting out) almost all of the remaining material.
Applications are repeated, usually on sequential nights, four times. A maximum of 16 times may be needed in some cases. The reason why it is used at night is that the patient is not moving and salivary flow is less at night.
When used in this way, very little of the gel comes into contact with tissues of the mouth other than the teeth and very little is swallowed.
The fact that Opalescence can only be sold to practising dentists ensures that it will only be used on patients for whom it is suitable and that over-frequent use and/or exposure does not occur. Similarly, the fact that only a dentist can provide the necessary customised tray ensures that the gums and other soft tissues of the mouth are only minimally exposed to the gel.
Before embarking on the use of Opalescence, the dentist needs to diagnose the cause of the discoloration and make sure that there is no tooth decay and no disease that would contraindicate such treatment. Pre-bleaching radiographs may be necessary to assess whether there is disease associated with a discoloured tooth (teeth) and also to see how close pulpal nerves are to the outer part of the tooth (teeth). A radiograph may also reveal fracture of the tooth root and indicate a need for other treatment before, or instead of, bleaching.
A dentist should assess any white fillings or crowns that a patient might have on teeth that are being considered for bleaching. He/she should tell the patient that these materials are unlikely to change colour during bleaching. In other words, patients often do not know which bit is the tooth and which the filling. They may not even know which teeth have been crowned. If restorations or crowns need to be changed for appearance reasons, this should be undertaken about 2 weeks after bleaching.
Rights and permissions
About this article
Cite this article
Kelleher, M., Roe, F. The safety-in-use of 10% carbamide peroxide (Opalescence) for bleaching teeth under the supervision of a dentist. Br Dent J 187, 190–194 (1999). https://doi.org/10.1038/sj.bdj.4800237
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.4800237
This article is cited by
-
Clinical use of hydrogen peroxide in surgery and dentistry – why is there a safety issue?
British Dental Journal (2010)