Abstract
We investigated prognostic factors for the clinical outcome of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) following imatinib-based therapy. Among 100 adult patients who were prospectively enrolled in the JALSG Ph+ALL202 study, 97 patients obtained complete remission (CR) by imatinib-combined chemotherapy, among whom 60 underwent allo-HSCT in their first CR. The probabilities of overall survival (OS) and disease-free survival (DFS) at 3 years after HSCT were 64% (95% CI, 49–76) and 58% (95% CI, 43–70), respectively. Prognostic factor analysis revealed that the major BCR–ABL transcript was the only unfavorable predictor for OS and DFS after HSCT by both univariate (HR, 3.67 (95% CI 1.49–9.08); P=0.005 and HR, 6.25 (95% CI, 1.88–20.8); P=0.003, respectively) and multivariate analyses (HR, 3.20 (95% CI, 1.21–8.50); P=0.019 and HR, 6.92 (95% CI, 2.09–22.9); P=0.002, respectively). Minimal residual disease status at the time of HSCT had a significant influence on relapse rate (P=0.015). Further study of the BCR–ABL subtype for the clinical impact on outcome of allo-HSCT in Ph+ALL is warranted.
Similar content being viewed by others
Introduction
Approximately 20 to 25% of adult patients with acute lymphoblastic leukemia (ALL) harbor BCR–ABL fusion gene. The prognosis following conventional chemotherapy of these patients had been extremely poor.1, 2, 3 Although the treatment of Philadelphia chromosome-positive ALL (Ph+ALL) has been changed dramatically since the introduction of imatinib,4 allogeneic hematopoietic stem cell transplantation (allo-HSCT) still seems to have a central role as a curative option for patients with Ph+ALL in the imatinib era.5, 6, 7 Previously we reported that the patients who had achieved complete remission (CR) by imatinib-based therapy, and subsequently received allo-HSCT in their first CR, showed significantly superior survival to those patients in the pre-imatinib era.8 Imatinib-based therapy is a useful strategy, giving patients not only a better chance to receive allo-HSCT but also improvement of the outcome after allo-HSCT. However, the treatment success after allo-HSCT is impaired by the occurrence of post-transplant relapse and non-relapse mortality (NRM),9, 10, 11 and therefore, identification of the risk factors causing relapse and NRM after allo-HSCT would be beneficial.
In the present study, we evaluated prognostic factors influencing overall survival (OS), disease-free survival (DFS), relapse and NRM after allo-HSCT among patients with Ph+ALL who underwent HSCT in the imatinib era, by using the prospectively conducted data of Japan Adult Leukemia Study Group (JALSG) Ph+ALL202 study.
Patients and Methods
Patients
In the JALSG Ph+ALL202 study, 100 newly diagnosed patients with BCR–ABL-positive ALL were registered consecutively between September 2002 and May 2005. All patients were diagnosed as Ph+ALL by real-time quantitative PCR (RQ-PCR) analysis, and received the same imatinib-combined chemotherapy, as described previously.12 Of 97 patients who achieved CR, 60 patients received allo-HSCT in their first CR. Table 1 shows the characteristics of these 60 patients analyzed in the present study. In the Ph+ALL202 study, allo-HSCT was recommended after achieving CR if a human leukocyte antigen (HLA)-identical donor was available. The stem-cell source for allo-HSCT was chosen in the following order: first, matched related donor; second, HLA-A, B and DRB1 allele matched (6/6) or DRB1 one-allele mismatched unrelated donor; and third, unrelated cord blood or HLA-mismatched related donor. Timing and procedure of HSCT, including conditioning regimen and graft-versus-host disease (GVHD) prophylaxis, were determined by each institution.
Among 60 patients, 32 were males and 28 females, with a median age of 37 years (range, 15–64 years), while 33 patients were less than 40. Regarding the BCR–ABL transcript types, two patients expressed both major and minor BCR–ABLs, and were categorized into the major BCR–ABL group in the subsequent analysis. Consequently, 42 patients were positive for minor BCR–ABL and 18 for major BCR–ABL. Pre-treatment cytogenetic results were not available for four patients because no analysis was performed (n=2) nor successful (n=2). Of the remaining 56 patients, 10 showed only t(9;22), 44 showed additional chromosome aberrations and 2 showed normal karyotype. Additional aberrations were comprised of +der (22) t(9;22) in 12 patients, del(9) in 3, monosomy 7 in 6 and trisomy 8 in 6. The study was approved by the institutional review board of each participating center and conducted in accordance with the Declaration of Helsinki.
Quantification of BCR–ABL Transcripts
The copy number of BCR–ABL transcripts in bone marrow was determined at the central laboratory using the RQ-PCR as described previously.12 To minimize the variability owing to differences in the efficiency of cDNA synthesis and RNA integrity among patient samples, the copy numbers of BCR–ABL transcripts were converted to molecules per microgram RNA after being normalized by GAPDH. The normalized values of the BCR–ABL copies in each sample were reported as the BCR–ABL number of copies. At least 5.7 × 105 copies/μg RNA GAPDH levels were required in a sample to be defined as a negative PCR result; otherwise, the sample was not used for minimal residual disease (MRD) studies. The threshold for quantification was 50 copies/μg RNA, which corresponded to a minimal sensitivity of 10−5. The levels below this threshold were designated as ‘not detected’ or ‘less than 50 copies/μg’, and the former was categorized as PCR negativity. MRD at the time of HSCT was evaluated by the result of RQ-PCR within 30 days before respective transplantation.
Statistical Considerations
The aim of this study was to identify prognostic factors for clinical outcome after allo-HSCT in patients with Ph+ALL transplanted in their CR in the imatinib era. Primary endpoint was OS after allo-HSCT, and secondary endpoints were NRM, relapse and DFS. OS was calculated from the date of transplantation to the date of death by any cause, or the last known date of follow-up. DFS was computed from the date of transplantation to the date of relapse, or death by any cause, or the last known date of follow-up. The probabilities of OS and DFS were estimated by Kaplan–Meier product limit method. Cumulative incidence of NRM, relapse, acute GVHD (aGVHD) and chronic GVHD (cGVHD) were estimated by the method taking the competing risks into account, as described elsewhere.13 In each estimation of cumulative incidence of events, death without an event was defined as a competing risk. Risk factors were evaluated by combination of uni- and multivariate analyses. We applied for univariate analysis Cox regression models or the log-rank test, and for multivariate analysis the Cox proportional hazards regression model or the competing risk regression model as appropriate.14
Covariates considered in uni- and multivariate analyses were: donor status, age at HSCT (<40, vs ⩾40), CD20 positivity (yes vs no), WBC counts at diagnosis (>30 × 109/l vs <30 × 109/l), additional chromosomal abnormality, stem-cell source (bone marrow, peripheral blood or cord blood), conditioning regimen (myeloablative vs reduced intensity), BCR–ABL subtype (major vs minor), performance status at HSCT (1–2 vs 0) and MRD at HSCT (PCR positive vs negative). Neutrophil recovery was defined by neutrophil counts of ⩾0.5 × 109/l in three consecutive days. Graft failure was defined as no sign of neutrophil recovery. aGVHD and cGVHD were defined according to previously described standard criteria.15
Results
Transplantation
Graft and conditioning regimen characteristics are summarized in Table 1. The median day from diagnosis to HSCT was 164 (range 67–512 days). One patient with no HLA-matched related donor received the scheduled therapy until a HLA-matched unrelated donor was available, and underwent HSCT at 512 days. The majority of donors were HLA-matched related (n=24) and unrelated (n=21), followed by mismatched unrelated cord blood (n=9) and mismatched related donors (n=6). Patients were treated with various conditioning regimens according to the transplant centers. The majority of patients (70%) received fractionated total body irradiation followed by cyclophosphamide and/or cytarabine. Six patients, older than 55, were given a reduced-intensity regimen consisting of fludarabine and melphalan or busulfan. No patient received imatinib therapy after HSCT. All patients who showed hematological relapse after HSCT received salvage treatment comprising of imatinib and/or chemotherapy.
The median days to reach a neutrophil count >0.5 × 109/l and platelet count ⩾50 × 109/l were 15 (range: 5–41 days) and 27 (range: 11–504 days), respectively. Cumulative incidence of grade 2 to 4 of aGVHD and of cGVHD at 1 year after HSCT were 33.3% (95% CI, 12–33%) and 44% (95% CI, 29–58%), respectively.
OS and DFS
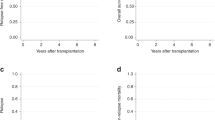
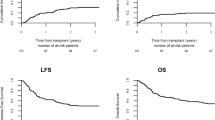
With a median follow-up of 31 months (range, 12 to 56) after HSCT, 41 patients were alive without relapse. The probability of OS and DFS at 3 years after HSCT were 64% (95% CI; 49–76%) and 58% (95% CI; 43–70%), respectively (Figure 1). By the uni- and multivariate analysis, the presence of major BCR–ABL transcript was only associated with unfavorable OS (HR=3.67 (95% CI, 1.49–9.08); P=0.005, and HR=6.25 (95% CI, 1.88–20.8); P=0.003, respectively) and DFS (HR=2.60 (95% CI, 1.16–5.83); P=0.02, and HR=3.20 (95% CI, 1.21–8.50); P=0.019, respectively) (Table 2). Figure 2 illustrates the 3-year OS and DFS in patients with major and minor BCR–ABL subtypes (37% vs 75%; P=0.003 and 33% vs 68%; P=0.016, respectively).
Relapse
Overall, 9 patients (15%) relapsed after HSCT, with a median day of 167 (range, 68–728 days). The estimated cumulative incidence of relapse at 3 years was 17% (95% CI, 8.3–28.0%). By the univariate analysis for relapse, PCR-negativity at HSCT (HR=4.82 (95% CI, 1.20–19.4); P=0.027) and peripheral blood as a stem-cell source (HR=5.53 (95% CI, 1.06–29.0); P=0.043) were associated with a lower relapse rate, but they did not reach statistical significance by the multivariate analysis (HR=7.34 (95% CI, 0.54–99.4); P=0.134 and HR=4.92 (95% CI, 0.17–144.0); P=0.355, respectively) (Table 3). The 3-year cumulative incidence of relapse rate was not different in patients with major and minor BCR–ABL subtypes (8% vs 20%; P=0.34).
NRM
Nineteen patients died after HSCT: 6 from relapsed ALL and 13 from causes other than leukemia. The causes of NRM included graft failure in 5, infection in 3, bronchiolitis obliterans in 2, cGVHD in 2 and unknown in 1. Estimated cumulative incidences of NRM at 3 years were 26% (95% CI, 14.8–38.7). By both uni- and multivariate analyses, the presence of major BCR–ABL transcript was associated with a higher NRM rate (HR=5.95 (95% CI, 2.06–17.2); P=0.001, and 6.92 (95% CI, 2.09–22.9), vs 0.002, respectively) (Table 3).
Figure 2 illustrates the 3-year cumulative incidence of NRM in patients with major and minor BCR–ABL subtypes (57% vs 13%; P=0.0004). Four patients (22%) with major BCR–ABL transcript, but only one (2%) with minor transcript, died from graft failure (Table 4).
Discussion
In the present study, in patients with Ph+ALL who had achieved CR by imatinib-based therapy and subsequently received allo-HSCT in their first CR, the major BCR–ABL subtype revealed significantly unfavorable prognostic impact on NRM, and consequently on OS and DFS (Figure 2). During the pre-imatinib era, several groups reported the relationship between the clinical outcome and BCR–ABL subtypes in patients with Ph+ALL. German Multicenter Adult ALL Study Group reported a trend toward poor OS for patients with major BCR–ABL (19% OS for the minor and 3% for the major at 3 years, P=0.07).2 Gruppo Italiano Malattie Ematologiche dell' Adulto also reported that minor BCR–ABL was an independent prognostic factor favorably affecting the 5-year OS and DFS (P=0.008 and P=0.02, respectively), although response rates to the induction therapy were similar in both groups.16 Of note in their study, none of 14 patients with major BCR–ABL transcript who underwent HSCT (8 allogeneic and 6 autologous) survived in CR, whereas, among 22 patients with minor BCR–ABL, 6 of 12 who received allo-HSCT and 2 of 10 who received autologous HSCT survived in CR.
In this imatinib era study, patients with major BCR–ABL transcript showed significantly unfavorable OS rates, compared with those with minor BCR–ABL transcript. Among 100 patients registered into the JALSG Ph+ALL 202 study, three patients died from chemotherapy-related toxicity during induction therapy and all of them expressed minor BCR–ABL transcript. Additionally, among 40 patients who did not receive HSCT in their first CR, OS of 7 patients with major BCR–ABL transcript was not inferior to that of 33 patients with minor BCR–ABL (P=0.254) (Supplemental figure S1). Therefore, the unfavorable clinical impact of major BCR–ABL transcript might be specific in the setting of allo-HSCT. Then the question arises: How the BCR–ABL subtype influenced the prognosis after allo-HSCT?
As shown in Table 4, MRD status and the period from diagnosis to HSCT were not significantly different among patients with major or minor BCR–ABL transcript. As the cause of NRM after allo-HSCT, high incidence of graft failure (22%) was observed in patients with major BCR–ABL (Table 4), and to predict NRM, transplantation-specific comorbidity index (HCT-CI) is reportedly useful.17 In the present study, 54 of 60 patients could be evaluable for this scoring system, but we found no difference in HCT-CI scores between major and minor BCR–ABL subtypes (P=0.40).
Biological heterogeneities between major and minor BCR–ABL transcripts may have influenced NRM of HSCT. Juric et al.18 performed a comprehensive analysis of the gene expression profiles in 37 BCR–ABL-positive adult ALL. They identified the genes overexpressed (PILRB, STS-1, SPRY1) or underexpressed (TSPAN16, ADAMTSL4) in ALL with minor BCR–ABL transcript, relative to ALL with major BCR–ABL, and constructed a gene expression- and interaction-based outcome predictor, consisting of 27 genes, which correlated with OS, independent of age and WBC count at presentation. Zheng et al.19 spotlighted the role of the reciprocal ABL–BCR fusion proteins, derivative chromosome 9 (der 9) -associated p96ABL–BCR and p40ABL–BCR fusion proteins. They indicated that p96ABL–BCR and p40ABL–BCR fusion proteins regulated the different expression of genes involved in the maintenance of stem-cell capacity. However, even if the biological heterogeneity would affect the clinical outcome of patients, the following question would arise: Could pre-existing aberrant gene translocation before allo-HSCT affect the prognosis of patients after transplantation? An inspiring report from Kreil et al.20 verifies a function of p40ABL–BCR fusion protein in the setting of allo-HSCT. They developed a DNA-based deletion screen, and investigated 339 patients with chronic phase CML and detected der (9) deletions in 59 (17%) patients. Of these, 21 spanned the ABL–BCR junction and 38 were centromeric or telomeric of the breakpoint. Patients with ABL–BCR junction-spanning deletions (p40ABL–BCR deficiency) had poorer survival, compared with patients without deletions.20 More interestingly, this tendency was most distinctive in the setting of allo-HSCT where bone marrow was replaced by normal stem cells from healthy donor.20 Deletions that did not span the ABL–BCR junction were associated with improved survival, compared with patients without deletions. From these, one could speculate that p40ABL–BCR has an important role on the stem-cell re-constitution after allo-HSCT in patients with BCR–ABL-positive leukemia, and that, even when the patient’s bone marrow was replaced by normal donor stem cells, a deficiency of this protein induced by imatinib-combined chemotherapy could contribute to the relatively high incidence of graft failure (22%) in patients with major BCR–ABL transcript as observed in our present study.
Investigation of transplant outcome of Ph+ALL patients who expressed minor BCR–ABL transcript and der (9) deletion would be helpful to evaluate clinical relevance of p96ABL–BCR. However, to our knowledge, there is no report focusing on the BCR–ABL subtypes and der (9) deletions in patients with Ph+ALL. In our present study, three patients who had der (9) deletions were all positive for minor BCR–ABL transcript and alive at the last known date of follow-up. Further investigation for clinico-biological effects of not only BCR–ABL but also ABL–BCR transcripts will be needed to clarify the prognostic relevance of BCR–ABL subtypes after allo-HSCT in patients with Ph+ALL.
We categorized two patients with both major and minor BCR–ABL transcripts into the major BCR–ABL transcript group. Several investigators who studied Ph+ALL with both BCR–ABL transcripts have reported that the level of minor BCR–ABL transcript was consistently low, such as only one transcript per 100 cells with major BCR–ABL transcript.21 Fujimaki et al.22 studied four patients with Ph+ALL with both transcripts before and after allo-HSCT, and reported that PCR negativity for minor BCR–ABL was documented in all cases 1–2 months before PCR negativity for major BCR–ABL. Taking these preceding studies into consideration, we believe our categorization of the two patients would be justified.
In the present study, negative MRD before HSCT resulted in significantly lower relapse rate after HSCT (Table 3). Some investigators reported that MRD before HSCT served as a powerful predictor of lower relapse rate and better DFS.4, 23, 24 Therefore, prospective monitoring of MRD may potentially identify patients at risk of relapse, although the implications of different transcript levels and increments require validation within each therapeutic context or clinical study.4 These issues highlight the need for the standardization and harmonization of methodologies used for BCR–ABL quantification in Ph+ALL.4 Employment of highly sensitive methods such as nested PCR or of normalization by total ABL transcripts may make clear the predictive value of MRD analysis for the prognosis after HSCT.25
To our knowledge, this is the first report on the clinical impact of the BCR–ABL subtypes on the outcomes of patients with Ph+ALL after allo-HSCT, analyzing results of a substantial number of patients with a sufficient follow-up period. However, the strength and limitations of our study need to be considered. The strength lies in the relatively large sample size, if not sufficient, and relatively homogenous population, as all patients received a uniform imatinib-combined chemotherapy regimen (JALSG Ph+ALL202)12 and underwent allo-HSCT in their first CR. These facts gave us a better estimation of the endpoints, and also added statistical power to the analyses. Our limitations are the presence of residual confounding factors, both known and unknown, and insufficient number of patients in each different prognostic factor. Among the known factors, difference in transplantation procedure, including pre-transplant conditioning regimens, should be noted. In this study, conditioning regimens and GVHD prophylaxis were determined by each institution. However, the small number of patients per institution and the changes of the conditioning regimens themselves within the same institution inevitably rendered the analysis on these factors impossible.
We have no comparative clinico-biological data in patients with Ph+ALL transplanted during the pre-imatinib era, and were unable to evaluate whether BCR–ABL subtype has a prognostic impact during that time. Further study should be undertaken to evaluate the prognostic value of BCR–ABL subtypes both in pre- and post imatinib eras.
The treatment strategy for Ph+ALL in the imatinib era, especially for Ph+ALL with major BCR–ABL transcript, should be reconsidered, and additionally, not only allo-HSCT but also second generation tyrosine kinase inhibitors need to be incorporated. Further study would be warranted to determine the clinical impact of BCR–ABL transcripts on the outcome of allo-HSCT in this disease.
References
Hoelzer D, Gokbuget N . Recent approaches in acute lymphoblastic leukemia in adults. Crit Rev Oncol Hematol 2000; 36: 49–58.
Gleissner B, Gokbuget N, Bartram CR, Janssen B, Rieder H, Janssen JW et alLeading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood 2002; 99: 1536–1543.
Takeuchi J, Kyo T, Naito K, Sao H, Takahashi M, Miyawaki S et alInduction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG-ALL93 study. Leukemia 2002; 16: 1259–1266.
Ottmann OG, Pfeifer H . Management of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). Hematol Am Soc Hematol Educ Program 2009, 371–381.
Ohno R . Changing paradigm of the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Curr Hematol Malig Rep 2010; 5: 213–221.
Lee S, Kim YJ, Min CK, Kim HJ, Eom KS, Kim DW et alThe effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood 2005; 105: 3449–3457.
Lee HJ, Thompson JE, Wang ES, Wetzler M . Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2011; 117: 1583–1594.
Mizuta S, Matsuo K, Yagasaki F, Yujiri T, Hatta Y, Kimura Y et alPre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia 2011; 25: 41–47.
Gruber F, Mustjoki S, Porkka K . Impact of tyrosine kinase inhibitors on patient outcomes in Philadelphia chromosome-positive acute lymphoblastic leukemia. Br J Haematol 2009; 145: 581–597.
Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I et alChemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group Protocol 09/00. J Clin Oncol 2010; 28: 3644–3652.
Mohty M, Labopin M, Volin L, Gratwohl A, Socié G, Esteve J et alReduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood 2010; 116: 4439–4443.
Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F et alHigh complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL–positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol 2006; 24: 460–466.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Fine JP, Gray RJ . A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Cimino G, Pane F, Elia L, Finolezzi E, Fazi P, Annino L et alThe role of BCR/ABL isoforms in the presentation and outcome of patients with Philadelphia-positive acute lymphoblastic leukemia: a seven-year update of the GIMEMA 0496 trial. Haematologica 2006; 91: 377–380.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et alHematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Juric D, Lacayo N, Ramsey M, Racevskis J, Wiernik PH, Rowe JM et alDifferential gene expression patterns and interaction networks in BCR-ABL–positive and –negative adult acute lymphoblastic leukemias. J Clin Oncol 2007; 25: 1341–1349.
Zheng X, Oancea C, Henschler R, Moore M, Ruthardt M . Reciprocal t(9;22) ABL/BCR fusion proteins: leukemogenic potential and effects on B cell commitment. PLoS One 2009; 10: e7661.
Kreil S, Pfirrmann M, Haferlach C, Waghorn K, Chase A, Hehlmann R et alHeterogeneous prognostic impact of derivative chromosome 9 deletions in chronic myelogenous leukemia. Blood 2007; 110: 1283–1290.
Rhee F, Hochhaus A, Lin F, Melo JV, Goldman JM, Cross NC . p190 BCR-ABL mRNA is expressed at low levels in p210-Positive chronic myeloid and acute lymphoblastic leukemias. Blood 1996; 87: 5213–5217.
Fujimaki K, Maruta A, Yoshida M, Yamazaki E, Motomura S, Kodama F et alSequential analysis of p210- and p190-bcr-abl by RT-PCR after allogeneic bone marrow transplantation for p210/p190-bcr-abl double positive acute lymphoblastic leukemia. Rinsho Ketsueki 2001; 42: 89–93.
Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X et alOutcome of treatment in adults with Philadelphia chromosome–positive acute lymphoblastic leukemia—results of the prospective multicenter LALA-94 trial. Blood 2002; 100: 2357–2366.
Lee S, Kim YJ, Chung NG, Lim J, Lee DG, Kim HJ et alThe extent of minimal residual disease reduction after the first 4-week imatinib therapy determines outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2009; 115: 561–570.
Guo JQ, Lin H, Kantarjian H, Talpaz M, Champlin R, Andreeff M et alComparison of competitive-nested PCR and real-time PCR in detecting BCR-ABL fusion transcripts in chronic myeloid leukemia patients. Leukemia 2002; 16: 2447–2453.
Acknowledgements
We thank Masamitsu Yanada, MD, and all the physicians and staff of the collaborating institutes of the Japan Adult Leukemia Study Group and Japan Society for Hematopoietic Cell Transplantation. We also thank Ryuzo Ohno, MD, for his assistance in the preparation of the manuscript. This work was supported by a Research Grant for Cancer from the Japanese Ministry of Health, Labor and Welfare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Naoe received research funding and honoraria from Novartis Japan. Dr Ohnishi received research funding from Novartis Japan. Dr Miyazaki received honoraria from Novartis Japan. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Mizuta, S., Matsuo, K., Maeda, T. et al. Prognostic factors influencing clinical outcome of allogeneic hematopoietic stem cell transplantation following imatinib-based therapy in BCR–ABL-positive ALL. Blood Cancer Journal 2, e72 (2012). https://doi.org/10.1038/bcj.2012.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2012.18
Keywords
This article is cited by
-
MRD in ALL: Optimization and Innovations
Current Hematologic Malignancy Reports (2022)
-
Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis
BMC Cancer (2018)
-
Final analysis of the JALSG Ph+ALL202 study: tyrosine kinase inhibitor-combined chemotherapy for Ph+ALL
Annals of Hematology (2018)
-
Impact of MRD and TKI on allogeneic hematopoietic cell transplantation for Ph+ALL: a study from the adult ALL WG of the JSHCT
Bone Marrow Transplantation (2016)
-
Clinical Outcome of Hematopoietic Stem Cell Transplantation for Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph + ALL): Experience From a Single Institution
Pathology & Oncology Research (2014)