Abstract
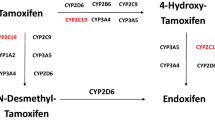
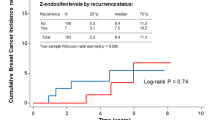
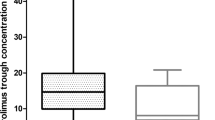
We investigated the impact of germline CYP2D6 genotyping done using the non-tumor specimen on endoxifen concentrations and/or clinical outcomes in breast cancer (BC) patients treated with tamoxifen in published studies. We evaluated published data from 13 001 patients in 29 studies. Mean±s.d. endoxifen concentrations were significantly lower in poor metabolizers (PM) versus extensive metabolizers (EM) (8.8±7.2 versus 22.3±11.8 ng ml−1; P<0.05). The PM status did not influence clinical outcomes in majority of the studies. However, only one study followed the Gaedigk activity scoring for phenotypic assignments, which predicted recurrence-free survival in CYP2D6 poor metabolizers. In two independent studies with 1676 patients, low endoxifen concentrations predicted poor BC-free survival. From our review of published data we found that standardization of CYP2D6 genotype-phenotype classification is needed in order to ensure effective evaluation of associations between CYP2D6 polymorphisms and endoxifen concentrations and BC outcomes. Universal implementation of this standardization classification system should be a priority among researchers and laboratories. Furthermore, additional clinical research is warranted to determine whether patients with CYP2D6 PM phenotypes or low endoxifen levels will have better clinical outcomes with increased tamoxifen dosing compared to standard dosing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29.
Clark GM, Osborne CK, McGuire WL . Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol 1984; 2: 1102–1109.
Osborne CK . Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat 1998; 51: 227–238.
Lumachi F, Santeufemia DA, Basso SM . Current medical treatment of estrogen receptor-positive breast cancer. World J Biol Chem 2015; 6: 231–239.
Wolmark N, Dunn BK . The role of tamoxifen in breast cancer prevention: issues sparked by the NSABP Breast Cancer Prevention Trial (P-1). Ann N Y Acad Sci 2001; 949: 99–108.
Vogel VG . Follow-up of the breast cancer prevention trial and the future of breast cancer prevention efforts. Clin Cancer Res 2001; 7 (12 Suppl): 4413s–4418ss, discussion 1–2s.
Markopoulos C, Kykalos S, Mantas D . Impact of CYP2D*6 in the adjuvant treatment of breast cancer patients with tamoxifen. World J Clin Oncol 2014; 5: 374–381.
Walko CM, McLeod H . Use of CYP2D6 genotyping in practice: tamoxifen dose adjustment. Pharmacogenomics 2012; 13: 691–697.
Desta Z, Ward BA, Soukhova NV, Flockhart DA . Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 2004; 310: 1062–1075.
Kim J, Lim YR, Han S, Han JS, Chun YJ, Yun CH et al. Functional influence of human CYP2D6 allelic variations: P34S, E418K, S486T, and R296C. Arch Pharm Res 2013; 36: 1500–1506.
Breast International Group 1-98 Collaborative G, Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005; 353: 2747–2757.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010; 11: 1135–1141.
Castells A, Gusella JF, Ramesh V, Rustgi AK . A region of deletion on chromosome 22q13 is common to human breast and colorectal cancers. Cancer Res 2000; 60: 2836–2839.
Hirano A, Emi M, Tsuneizumi M, Utada Y, Yoshimoto M, Kasumi F et al. Allelic losses of loci at 3p25.1, 8p22, 13q12, 17p13.3, and 22q13 correlate with postoperative recurrence in breast cancer. Clin Cancer Res 2001; 7: 876–882.
Loo LW, Ton C, Wang YW, Grove DI, Bouzek H, Vartanian N et al. Differential patterns of allelic loss in estrogen receptor-positive infiltrating lobular and ductal breast cancer. Genes Chromosomes Cancer 2008; 47: 1049–1066.
Nakamura Y, Ratain MJ, Cox NJ, McLeod HL, Kroetz DL, Flockhart DA . Re: CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1-98 trial. J Natl Cancer Inst 2012; 104: 1264 author reply 6-8.
Goetz MP, Sun JX, Suman VJ, Silva GO, Perou CM, Nakamura Y et al. Loss of heterozygosity at the CYP2D6 locus in breast cancer: implications for germline pharmacogenetic studies. J Natl Cancer Inst 2014; 107: dju401.
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS . The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 2008; 83: 234–242.
Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther 2014; 95: 376–382.
Abraham JE, Maranian MJ, Driver KE, Platte R, Kalmyrzaev B, Baynes C et al. CYP2D6 gene variants: association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen. Breast Cancer Res 2010; 12: R64.
Barginear MF, Jaremko M, Peter I, Yu C, Kasai Y, Kemeny M et al. Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Ther 2011; 90: 605–611.
Bijl MJ, van Schaik RH, Lammers LA, Hofman A, Vulto AG, van Gelder T et al. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat 2009; 118: 125–130.
Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 2006; 80: 61–74.
Damodaran SE, Pradhan SC, Umamaheswaran G, Kadambari D, Reddy KS, Adithan C . Genetic polymorphisms of CYP2D6 increase the risk for recurrence of breast cancer in patients receiving tamoxifen as an adjuvant therapy. Cancer Chemother Pharmacol 2012; 70: 75–81.
Irvin WJ Jr., Walko CM, Weck KE, Ibrahim JG, Chiu WK, Dees EC et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: a multicenter study. J Clin Oncol 2011; 29: 3232–3239.
Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol 2010; 28: 1287–1293.
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 2011; 89: 718–725.
Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther 2011; 89: 708–717.
Opdam FL, Dezentje VO, den Hartigh J, Modak AS, Vree R, Batman E et al. The use of the 13C-dextromethorphan breath test for phenotyping CYP2D6 in breast cancer patients using tamoxifen: association with CYP2D6 genotype and serum endoxifen levels. Cancer Chemother Pharmacol 2013; 71: 593–601.
Park IH, Ro J, Park S, Lim HS, Lee KS, Kang HS et al. Lack of any association between functionally significant CYP2D6 polymorphisms and clinical outcomes in early breast cancer patients receiving adjuvant tamoxifen treatment. Breast Cancer Res Treat 2012; 131: 455–461.
Ruddy KJ, Desantis SD, Gelman RS, Wu AH, Punglia RS, Mayer EL et al. Personalized medicine in breast cancer: tamoxifen, endoxifen, and CYP2D6 in clinical practice. Breast Cancer Res Treat 2013; 141: 421–427.
Saladores P, Murdter T, Eccles D, Chowbay B, Zgheib NK, Winter S et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J 2015; 15: 84–94.
Teh LK, Mohamed NI, Salleh MZ, Rohaizak M, Shahrun NS, Saladina JJ et al. The risk of recurrence in breast cancer patients treated with tamoxifen: polymorphisms of CYP2D6 and ABCB1. AAPS J 2012; 14: 52–59.
Margolin S, Lindh JD, Thoren L, Xie H, Koukel L, Dahl ML et al. CYP2D6 and adjuvant tamoxifen: possible differences of outcome in pre- and post-menopausal patients. Pharmacogenomics 2013; 14: 613–622.
Markkula A, Hjertberg M, Rose C, Ingvar C, Jernstrom H . No association found between CYP2D6 genotype and early breast cancer events in tamoxifen-treated patients. Acta Oncol 2014; 53: 195–200.
Okishiro M, Taguchi T, Jin Kim S, Shimazu K, Tamaki Y, Noguchi S . Genetic polymorphisms of CYP2D6 10 and CYP2C19 2, 3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer 2009; 115: 952–961.
Park HS, Choi JY, Lee MJ, Park S, Yeo CW, Lee SS et al. Association between genetic polymorphisms of CYP2D6 and outcomes in breast cancer patients with tamoxifen treatment. J Korean Med Sci 2011; 26: 1007–1013.
Goetz MP, Schaid DJ, Wickerham DL, Safgren S, Mushiroda T, Kubo M et al. Evaluation of CYP2D6 and efficacy of tamoxifen and raloxifene in women treated for breast cancer chemoprevention: results from the NSABP P1 and P2 clinical trials. Clin Cancer Res 2011; 17: 6944–6951.
Brooks JD, Teraoka SN, Malone KE, Haile RW, Bernstein L, Lynch CF et al. Variants in tamoxifen metabolizing genes: a case-control study of contralateral breast cancer risk in the WECARE study. Int J Mol Epidemiol Genet 2013; 4: 35–48.
Sirachainan E, Jaruhathai S, Trachu N, Panvichian R, Sirisinha T, Ativitavas T et al. CYP2D6 polymorphisms influence the efficacy of adjuvant tamoxifen in Thai breast cancer patients. Pharmgenomics Pers Med 2012; 5: 149–153.
Lim HS, Ju Lee H, Seok Lee K, Sook Lee E, Jang IJ, Ro J . Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol 2007; 25: 3837–3845.
Love RR, Desta Z, Flockhart D, Skaar T, Ogburn ET, Ramamoorthy A et al. CYP2D6 genotypes, endoxifen levels, and disease recurrence in 224 Filipino and Vietnamese women receiving adjuvant tamoxifen for operable breast cancer. Springerplus 2013; 2: 52.
Newman WG, Hadfield KD, Latif A, Roberts SA, Shenton A, McHague C et al. Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res 2008; 14: 5913–5918.
Nowell SA, Ahn J, Rae JM, Scheys JO, Trovato A, Sweeney C et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat 2005; 91: 249–258.
Ramon y Cajal T, Altes A, Pare L, del Rio E, Alonso C, Barnadas A et al. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat 2010; 119: 33–38.
Sestak I, Kealy R, Nikoloff M, Fontecha M, Forbes JF, Howell A et al. Relationships between CYP2D6 phenotype, breast cancer and hot flushes in women at high risk of breast cancer receiving prophylactic tamoxifen: results from the IBIS-I trial. Br J Cancer 2012; 107: 230–233.
Mwinyi J, Vokinger K, Jetter A, Breitenstein U, Hiller C, Kullak-Ublick GA et al. Impact of variable CYP genotypes on breast cancer relapse in patients undergoing adjuvant tamoxifen therapy. Cancer Chemother Pharmacol 2014; 73: 1181–1188.
Sukasem C, Sirachainan E, Chamnanphon M, Pechatanan K, Sirisinha T, Ativitavas T et al. Impact of CYP2D6 polymorphisms on tamoxifen responses of women with breast cancer: a microarray-based study in Thailand. Asian Pac J Cancer Prev 2012; 13: 4549–4553.
Ahern TP, Hertz DL, Damkier P, Ejlertsen B, Hamilton-Dutoit SJ, Rae JM et al. Cytochrome P-450 2D6 (CYP2D6) genotype and breast cancer recurrence in tamoxifen-treated patients: evaluating the importance of loss of heterozygosity. Am J Epidemiol 2017; 185: 75–85.
Acknowledgements
This paper was presented at 2016 Hematology/Oncology Pharmacy Association (HOPA) Annual Conference in Atlanta, GA and at 2016 American College of Clinical Pharmacy (ACCP) Annual Conference in Hollywood, FL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Hwang, G., Bhat, R., Crutchley, R. et al. Impact of CYP2D6 polymorphisms on endoxifen concentrations and breast cancer outcomes. Pharmacogenomics J 18, 201–208 (2018). https://doi.org/10.1038/tpj.2017.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2017.36
This article is cited by
-
Therapeutic Drug Monitoring of Endoxifen for Tamoxifen Precision Dosing: Feasible in Patients with Hormone-Sensitive Breast Cancer
Clinical Pharmacokinetics (2022)
-
Pharmacogenetics of tamoxifen therapy in Asian populations: from genetic polymorphism to clinical outcomes
European Journal of Clinical Pharmacology (2021)
-
Pharmacogenomics of breast cancer: highlighting CYP2D6 and tamoxifen
Journal of Cancer Research and Clinical Oncology (2020)
-
The Effect of Selenium on CYP450 Isoform Activity and Expression in Pigs
Biological Trace Element Research (2020)