Abstract
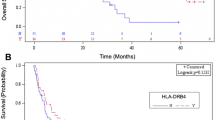
Lapatinib enhances antibody-dependent cell-mediated cytotoxicity (ADCC) activity of trastuzumab. FcγR polymorphisms have been associated with both ADCC and clinical activity of trastuzumab in HER2+ breast cancer (BC) patients (pts). We analyzed FcγRIIa-H131R and FcγRIIIa-V158F polymorphisms in the CHER-LOB trial population of HER2+ BCs treated with preoperative chemotherapy plus trastuzumab (arm A), lapatinib (arm B) or both (arm C). Genotyping was successfully performed in 73/121 (60%) pts. A significant improvement in pathological complete response (pCR) rate was observed for the combination arm C, but only in FcγRIIIa V allele carriers (C vs A, 67 vs 27%, P=0.043; C vs B, 67 vs 22%, P=0.012). An independent interaction between arm C and FcγRIIIa V allele was found for pCR (odds ratio=9.4; 95% confidence interval, 2.3–39.6; P=0.003). No significant associations were observed between pCR and FcγRIIa polymorphism, and between pre-treatment tumor-infiltrating lymphocytes and FcγR polymorphisms. Our study provides evidence for a FcγRIIIa V allele-restricted pCR benefit from neoadjuvant trastuzumab plus lapatinib in HER2+ BC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–182.
Musolino A, Boggiani D, Sikokis A, Rimanti A, Pellegrino B, Vattiato R et al. Prognostic risk factors for treatment decision in pT1a,b N0M0 HER2-positive breast cancers. Cancer Treat Rev 2016; 43: 1–7.
Slamon DJ, Eiermann W, Robert NJ, Giermek J, Martin M, Jasiowka M et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res 2015; 75 (Suppl 24): 5169 (abstract S5-04).
Lavaud P, Andre F . Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med 2014; 12: 132.
Montemurro F, Scaltriti M . Biomarkers of drugs targeting HER-family signalling in cancer. J Pathol 2014; 232: 219–229.
Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL . Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 2002; 62: 4132–4141.
Lu Y, Zi X, Zhao Y, Pollak M . Overexpression of ErbB2 receptor inhibits IGF-I-induced Shc-MAPK signaling pathway in breast cancer cells. Biochem Biophys Res Commun 2004; 313: 709–715.
Dubská L, Andera L, Sheard MA . HER2 signaling downregulation by Trastuzumab and suppression of the PI3K/Akt pathway: an unexpected effect on TRAIL-induced apoptosis. FEBS Lett 2005; 579: 4149–4158.
Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of Trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26: 1789–1796.
Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D . ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer 2002; 94: 2855–2861.
Baselga J, Cortés J, Im SA, Clark E, Ross G, Kiermaier A et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol 2014; 32: 3753–3761.
Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates Trastuzumab-dependent cell cytotoxicity. Oncogene 2009; 28: 803–814.
Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 2006; 94: 259–267.
Robertson MJ, Ritz J . Biology and relevance of human natural killer cells. Blood 1990; 76: 2421–2438.
Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M . Fc-gamma-RIIIa-158 V/F polymorphism influences the binding of IgG by natural killer cell Fc-gamma-RIIIa, independently of the Fc-gamma-RIIIa-48 L/R/H phenotype. Blood 1997; 90: 1109–1114.
Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improves binding to the Fc gamma R. J. Biol Chem 2001; 9: 6591–6604.
Negri FV, Musolino A, Naldi N, Bortesi B, Missale G, Laccabue D et al. Role of immunoglobulin G fragment C receptor polymorphism-mediated antibody-dependent cellular cytotoxicity in colorectal cancer treated with cetuximab therapy. Pharmacogenomics J 2014; 14: 14–19.
Tamura K, Shimizu C, Hojo T, Akashi-Tanaka S, Kinoshita T, Yonemori K et al. FcgammaR2A and 3 A polymorphisms predict clinical outcome of Trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol 2011; 22: 1302–1307.
Hurvitz SA, Betting DJ, Stern HM, Quinaux E, Stinson J, Seshagiri S et al. Analysis of Fcgamma receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin Cancer Res 2012; 18: 3478–3486.
Norton N, Olson RM, Pegram M, Tenner K, Ballman KV, Clynes R et al. Association studies of Fcγ receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831. Cancer Immunol Res 2014; 2: 962–969.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C et al. Lapatinib with Trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2012; 379: 633–640.
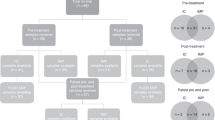
Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S et al. Pre-operative chemotherapy plus Trastuzumab, Lapatinib, or both in Epidermal Growth Factor 2-positive operable breast cancer: results of the randomized phase II study CHER-LOB. J Clin Oncol 2012; 30: 1989–1995.
Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 2014; 25: 611–618.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILsWorking Group 2014. Ann Oncol 2015; 26: 259–271.
Dieci MV, Bisagni G, Cagossi K, Bottini A, Sarti S, Piacentini F et al. Tumor infiltrating lymphocytes and correlation with outcome in the Cher-LOB study. Cancer Res 2015; 75 (Suppl 24): 5170 (abstract PD1-1).
Chernoff H, Lehmann EL . The use of maximum likelihood estimates in χ2 tests for goodness-of-fit. Ann Math Stat 1954; 25: 579–586.
Freeman DH Applied Categorical Data Analysis. Marcel Dekker, Inc.: New York, NY, USA, 1987.
Hardy GH . Mendelian proportions in a mixed population. Science 1908; 28: 49–50.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Mantel N . Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–170.
Cox DR . Regression models and life tables. J R Stat Soc 1972; 34: 187–220.
Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015; 372: 724–734.
Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012; 30: 2585–2592.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13: 25–32.
Piccart-Gebhart MJ, Holmes AP, Baselga J, De Azambuja E, Dueck AC, Viale G et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2–positive breast cancer: results from the randomized phase iii adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol 2015; 34: 1034–1042.
US National Institutes of Health: a study of Pertuzumab in Addition to Chemotherapy and Herceptin (trastuzumab) As Adjuvant Therapy in Patients with HER2-Positive Primary Breast Cancer. Available at https://clinicaltrials.gov/show/NCT01358877. Accessed 18 January 2016.
US National Institutes of Health: a study of Kadcyla (trastuzumab emtansine) Plus Perjeta (pertuzumab) Following Anthracyclines in Comparison with Herceptin (trastuzumab) Plus Perjeta and a Taxane Following Anthracyclines As Adjuvant Therapy in Patients With Operable HER2-Positive Primary Breast Cancer. Available at https://clinicaltrials.gov/show/NCT01966471. Accessed 18 January 2016.
Perez EA, Romond EH, Suman VJ, Jeong J-H, Davidson NE, Geyer CE et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-postive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011; 29: 3366–3373.
Guarneri V, Dieci MV, Frassoldati A, Maiorana A, Ficarra G, Bettelli S et al. Prospective biomarker analysis of the randomized CHER-LOB study evaluating the dual Anti-HER2 treatment with trastuzumab and lapatinib plus chemotherapy as neoadjuvant therapy for HER2-positive breast cancer. Oncologist 2015; 20: 1001–1010.
Lejeune J, Thibault G, Ternant D, Cartron G, Watier H, Ohresser M . Evidence for linkage disequilibrium between Fcgamma RIIIa-V158F and Fcgamma RIIa-H131R polymorphisms in white patients, and for an Fcgamma RIIIa-restricted influence on the response to therapeutic antibodies. J Clin Oncol 2008; 26: 5489–5491.
Salgado R, Denkert C, Campbell C, Savas P, Nucifero P, Aura C et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 2015; 1: 448–454.
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2–positive and triple-negative primary breast cancers. J Clin Oncol 2015; 33: 983–991.
Zhu EF, Gai SA, Opel CF, Kwan BH, Surana R, Mihm MC et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell 2015; 27: 489–501.
Perez EA, Thompson EA, Ballman KV, Anderson SK, Asmann YW, Kalari KR et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the north central cancer treatment group n9831 adjuvant trastuzumab trial. J Clin Oncol 2015; 33: 701–708.
Scaltriti M, Nuciforo P, Bradbury I, Sperinde J, Agbor-Tarh D, Campbell C et al. High HER2 expression correlates with response to the combination of lapatinib and trastuzumab. Clin Cancer Res 2015; 21: 569–576.
Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells In vitro and controls tumor expansion in vivo via low-affinity activating fc receptors. Cancer Res 2007; 67: 8882–8890.
Acknowledgements
This study was supported, in part, by a grant from PGxHealth LLC/Transgenomic, which also provided technical assistance for FcγRIIa/IIIa genotype analyses. We would like to thank all participating patients and staff at CHER-LOB trial sites.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Musolino, A., Naldi, N., Dieci, M. et al. Immunoglobulin G fragment C receptor polymorphisms and efficacy of preoperative chemotherapy plus trastuzumab and lapatinib in HER2-positive breast cancer. Pharmacogenomics J 16, 472–477 (2016). https://doi.org/10.1038/tpj.2016.51
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2016.51
This article is cited by
-
Immunogenicity of rat-neu+ mouse mammary tumours determines the T cell-dependent therapeutic efficacy of anti-neu monoclonal antibody treatment
Scientific Reports (2020)
-
Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond
npj Breast Cancer (2020)
-
Biological Characterization of SB3, a Trastuzumab Biosimilar, and the Influence of Changes in Reference Product Characteristics on the Similarity Assessment
BioDrugs (2019)