Abstract
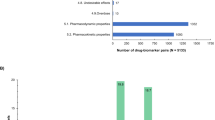
Regulatory agencies are increasing the pharmacogenomic information in their official drug labeling. However, despite the importance of regulatory harmonization, this implementation may not be running in parallel among major agencies. Comparing labeling of medicines approved by different agencies may identify gaps to solve. Our study compared the cytochrome P450 pharmacogenetic information included in the United States (US) Food and Drug Administration (FDA) drug labels and European Union (EU) Summaries of Product Characteristics (SmPCs). US labels presented significantly more specific pharmacogenetic subheadings (51 vs 26%), more prevalence and pharmacokinetic data for each metabolic phenotype (59 vs 25% and 82 vs 48%, respectively) and more applicable information about dose modifications required (25 vs 5%). Approximately 75% of the US labels evaluated scored higher on the overall quality than the analogous EU SmPCs, and this difference was not associated with the time since the EU SmPCs' last review. To enhance harmonization, regulatory agencies should simultaneously introduce the pharmacogenetic information in their drug labeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W . Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA 2001; 286: 2270–2279.
Bertilsson L . Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet 1995; 29: 192–209.
de Leon J, Susce MT, Murray-Carmichael E . The AmpliChip CYP450 genotyping test: integrating a new clinical tool. Mol Diagn Ther 2006; 10: 135–151.
Food and Drug Administration. Guidance for Industry Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling. 2013; Available at http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm337169.pdf (accessed 1 July 2015).
Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labels. Available at http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm (accessed 1 July 2015).
Zineh I, Gerhard T, Aquilante CL, Beitelshees AL, Beasley BN, Hartzema AG . Availability of pharmacogenomics-based prescribing information in drug package inserts for currently approved drugs. Pharmacogenomics J 2004; 4: 354–358.
Zineh I, Pebanco GD, Aquilante CL, Gerhard T, Beitelshees AL, Beasley BN et al. Discordance between availability of pharmacogenetics studies and pharmacogenetics-based prescribing information for the top 200 drugs. Ann Pharmacother 2006; 40: 639–644.
Frueh FW, Amur S, Mummaneni P, Epstein RS, Aubert RE, DeLuca TM et al. Pharmacogenomic biomarker information in drug labels approved by the United States food and drug administration: prevalence of related drug use. Pharmacotherapy 2008; 28: 992–998.
Otsubo Y, Asahina Y, Noguchi A, Sato Y, Ando Y, Uyama Y . Similarities and differences between US and Japan as to pharmacogenomic biomarker information in drug labels. Drug Metab Pharmacokinet 2012; 27: 142–149.
Conrado DJ, Rogers HL, Zineh I, Pacanowski MA . Consistency of drug-drug and gene-drug interaction information in US FDA-approved drug labels. Pharmacogenomics 2013; 14: 215–223.
Shimazawa R, Ikeda M . Differences in pharmacogenomic biomarker information in package inserts from the United States, the United Kingdom and Japan. J Clin Pharm Ther 2013; 38: 468–475.
Overby CL, Tarczy-Hornoch P, Hoath JI, Kalet IJ, Veenstra DL . Feasibility of incorporating genomic knowledge into electronic medical records for pharmacogenomic clinical decision support. BMC Bioinformatics 2010; 11: S10.
European Commission. Notice to Applicants. A guideline on Summary of Product Characteristics (SmPC). 2009; Available at http://ec.europa.eu/health/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf (accessed 1 July 2015).
European Medicines Agency. How to prepare and review a Summary of Product Characteristics. 2013; Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000357.jsp&mid=WC0b01ac05806361e1 (accessed 1 July 2015).
European Medicines Agency. Guideline on the use of pharmacogenetic methodologies in the pharmacokinetic evaluation of medicinal products. 2011; Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/02/WC500121954.pdf (accessed 1 July 2015).
Ehmann F, Caneva L, Prasad K, Paulmichl M, Maliepaard M, Llerena A et al. Pharmacogenomic information in drug labels: European Medicines Agency perspective. Pharmacogenomics J 2015; 15: 201–210.
San Miguel MT, Martinez JA, Vargas E . Food-drug interactions in the summary of product characteristics of proprietary medicinal products. Eur J Clin Pharmacol 2005; 61: 77–83.
Ferner RE, Coleman J, Pirmohamed M, Constable SA, Rouse A . The quality of information on monitoring for haematological adverse drug reactions. Br J Clin Pharmacol 2005; 60: 448–451.
Bergk V, Haefeli WE, Gasse C, Brenner H, Martin-Facklam M . Information deficits in the summary of product characteristics preclude an optimal management of drug interactions: a comparison with evidence from the literature. Eur J Clin Pharmacol 2005; 61: 327–335.
Arguello B, Fernandez-Llimos F . Clinical pharmacology information in summaries of product characteristics and package inserts. Clin Pharmacol Ther 2007; 82: 566–571.
Wall AJ, Bateman DN, Waring WS . Variability in the quality of overdose advice in Summary of Product Characteristics (SPC) documents: gut decontamination recommendations for CNS drugs. Br J Clin Pharmacol 2009; 67: 83–87.
Rougemont M, Ulrich S, Hiemke C, Corruble E, Baumann P . French summaries of product characteristics: content in relation to therapeutic monitoring of psychotropic drugs. Fundam Clin Pharmacol 2010; 24: 377–384.
Geerts AF, De Koning FH, Van Solinge WW, De Smet PA, Egberts TC . Instructions on laboratory monitoring in 200 drug labels. Clin Chem Lab Med 2012; 50: 1351–1358.
Beers E, Egberts TC, Leufkens HG, Jansen PA . Information for adequate prescribing to older patients: an evaluation of the product information of 53 recently approved medicines. Drugs Aging 2013; 30: 255–262.
Salgado TM, Arguello B, Martinez-Martinez F, Benrimoj SI, Fernandez-Llimos F . Clinical relevance of information in the Summaries of Product Characteristics for dose adjustment in renal impairment. Eur J Clin Pharmacol 2013; 69: 1973–1979.
Arguello B, Salgado TM, Fernandez-Llimos F . Assessing the information in the Summaries of Product Characteristics for the use of medicines in pregnancy and lactation. Br J Clin Pharmacol 2015; 79: 537–544.
Maxwell S, Eichler HG, Bucsics A, Haefeli WE, Gustafsson LL . e-SPC - delivering drug information in the 21st century: developing new approaches to deliver drug information to prescribers. Br J Clin Pharmacol 2012; 73: 12–15.
Fernandez-Llimos F . Quality of drug information for healthcare professionals: the ARCA acronym. Pharm Pract (Granada) 2015; 13: 709.
Spyker DA, Harvey ED, Harvey BE, Harvey AM, Rumack BH, Peck CC et al. Assessment and reporting of clinical pharmacology information in drug labeling. Clin Pharmacol Ther 2000; 67: 196–200.
Sim J, Wright CC . The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 2005; 85: 257–268.
Abramson JH . WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov 2011; 8: 1.
Raynor DK, Veene PD, Bryant D . The effectiveness of the Summary of Product Characteristics (SmPC) and recommendations for improvement. Therap Innov Regul Sci 2014; 48: 255–265.
Vromans L, Doyle G, Petak-Opel S, Rodiger A, Rottgermann M, Schlussel E et al. Shaping medicinal product information: a before and after study exploring physicians' perspectives on the summary of product characteristics. BMJ Open 2013; 3: e003033.
Quinzler R, Gasse C, Schneider A, Kaufmann-Kolle P, Szecsenyi J, Haefeli WE . The frequency of inappropriate tablet splitting in primary care. Eur J Clin Pharmacol 2006; 62: 1065–1073.
Arnet I, Hersberger KE . Misleading score-lines on tablets: facilitated intake or fractional dosing? Swiss Med Wkly 2010; 140: 105–110.
Larsson I, Kart T . Evaluation of sources to document extended shelf lives of compounded cytostatics. J Oncol Pharm Pract 2013; 19: 355–364.
Salgado TM, Arguello B, Martinez-Martinez F, Benrimoj SI, Fernandez-Llimos F . Lack of harmonisation in the classification of renal impairment in European Summaries of Product Characteristics. Intern Med J 2015; 45: 686–687.
Johnson JA, Burkley BM, Langaee TY, Clare-Salzler MJ, Klein TE, Altman RB . Implementing personalized medicine: development of a cost-effective customized pharmacogenetics genotyping array. Clin Pharmacol Ther 2012; 92: 437–439.
Vivot A, Boutron I, Ravaud P, Porcher R . Guidance for pharmacogenomic biomarker testing in labels of FDA-approved drugs. Genet Med 2014; 17: 733–738.
Gardiner SJ, Begg EJ . Pharmacogenetic testing for drug metabolizing enzymes: is it happening in practice? Pharmacogenet Genomics 2005; 15: 365–369.
Shah RR, Smith RL . Addressing phenoconversion: the Achilles' heel of personalized medicine. Br J Clin Pharmacol 2015; 79: 222–240.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Reis-Pardal, J., Rodrigues, A., Rodrigues, E. et al. Comparing cytochrome P450 pharmacogenetic information available on United States drug labels and European Union Summaries of Product Characteristics. Pharmacogenomics J 17, 488–493 (2017). https://doi.org/10.1038/tpj.2016.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2016.40
This article is cited by
-
Pharmacogenomic biomarker information on drug labels of the Spanish Agency of Medicines and Sanitary products: evaluation and comparison with other regulatory agencies
The Pharmacogenomics Journal (2024)
-
Pharmacogenetic information in Swiss drug labels – a systematic analysis
The Pharmacogenomics Journal (2021)
-
Pharmacogenomic biomarker information differences between drug labels in the United States and Hungary: implementation from medical practitioner view
The Pharmacogenomics Journal (2020)